Similar Questions
Explore conceptually related problems
Recommended Questions
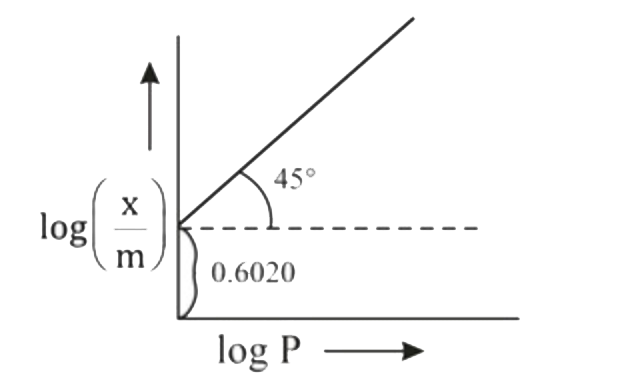
- Graph between log((x)/(m)) and log P is straight line at angle of 45^(...
Text Solution
|
- Graph between log x//m and log p is a straight line inclined at an ang...
Text Solution
|
- When a graph is plotted between log x//m and log p, it is striaght lin...
Text Solution
|
- A graph between log (x/m) and log p is straight line at an angle of 45...
Text Solution
|
- Graph between log x/m and log P is a straight line at angle of 45^(@) ...
Text Solution
|
- When a graph is plotted between log x/m and log p, it is straight lin...
Text Solution
|
- When a graph is pplotted between log x//m and log p, it is straight li...
Text Solution
|
- Graph between log ((x)/(m)) and log p is a straight line at an angle 4...
Text Solution
|
- Graph between log((x)/(m)) and log P is straight line at angle of 45^(...
Text Solution
|