Similar Questions
Explore conceptually related problems
Recommended Questions
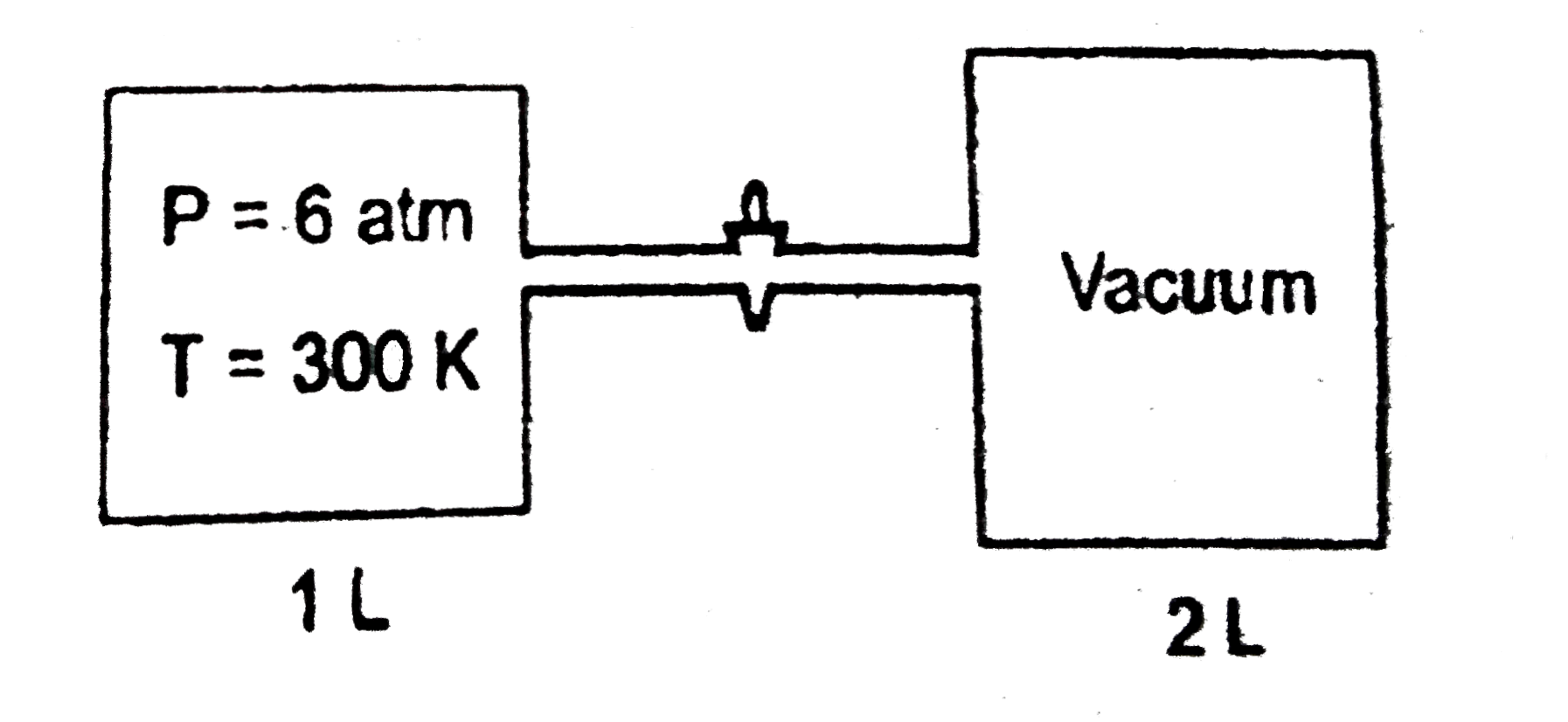
- It is known fact that gases expand to fill all available volume and th...
Text Solution
|
- The figure given below shows three glass chambers that are connected b...
Text Solution
|
- A quantity of heat is confined in a chamber of constant volume. When t...
Text Solution
|
- The molar gas constant is the same for all gases because at the same t...
Text Solution
|
- A gas at temperature 270^(0)C and pressure 30 atmosphere is allowed to...
Text Solution
|
- It is known fact that gases expand to fill all available volume and th...
Text Solution
|
- It is known fact that gases expand to fill all available volume and th...
Text Solution
|
- Assertion : Equal volumes of all gases under similar conditions of tem...
Text Solution
|
- A gas at temperature 27^@C and pressure 30 atm is allowed to expand to...
Text Solution
|