Similar Questions
Explore conceptually related problems
Recommended Questions
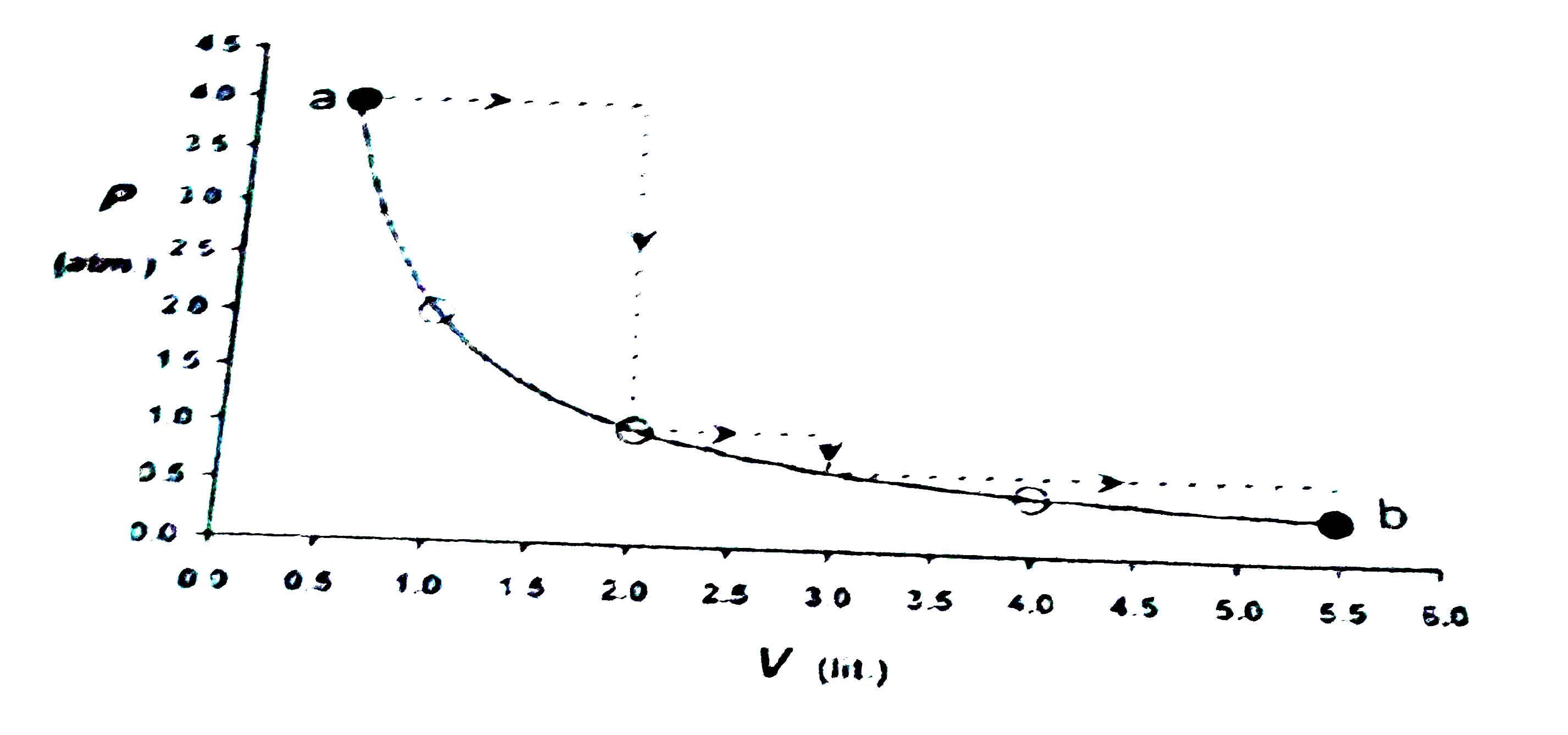
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- A given mass of gas expands from state A to state b by three paths 1,2...
Text Solution
|
- A given mass of a gas expands from the state A to the state B by three...
Text Solution
|
- One mole of an ideal gas is taken from a to b along two parths dented ...
Text Solution
|
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- One mole of an ideal gas is taken from a and b along two paths denoted...
Text Solution
|
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- A given mass of gas expands from state A to state B by three paths 1, ...
Text Solution
|
- One mole of an ideal gas is taken from a and b along two paths denoted...
Text Solution
|