Similar Questions
Explore conceptually related problems
Recommended Questions
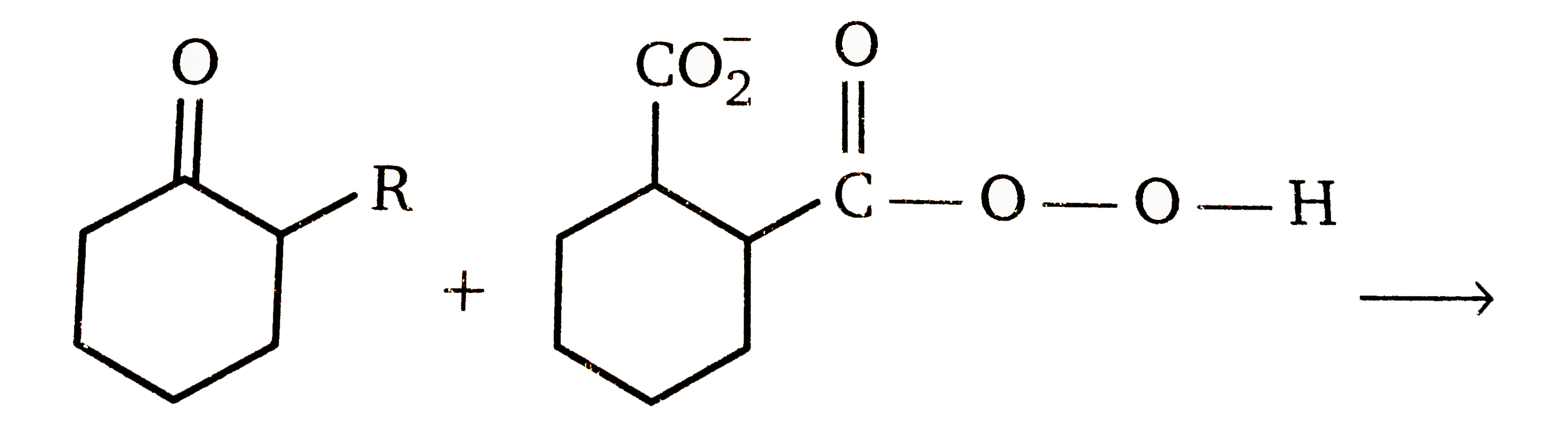
- Above reaction is a Baeyer Villiger rearrangement of an asymmetric ket...
Text Solution
|
- Consider the following Baeyer-Villiger oxidation.
Text Solution
|
- Select the correct Baeyer-Villiger oxidation reaction:
Text Solution
|
- BAEYER-VILLIGER REACTION
Text Solution
|
- Identify the major products of the above reaction ?
Text Solution
|
- Oxidation of ketones with H(2)O(2) or with a peroxy acid is called Bae...
Text Solution
|
- Above reaction is a Baeyer Villiger rearrangement of an asymmetric ket...
Text Solution
|
- Identify the product of the above rearrangement reaction.
Text Solution
|
- The major mono product in the reaction is X. Identify the product...
Text Solution
|