Similar Questions
Explore conceptually related problems
Recommended Questions
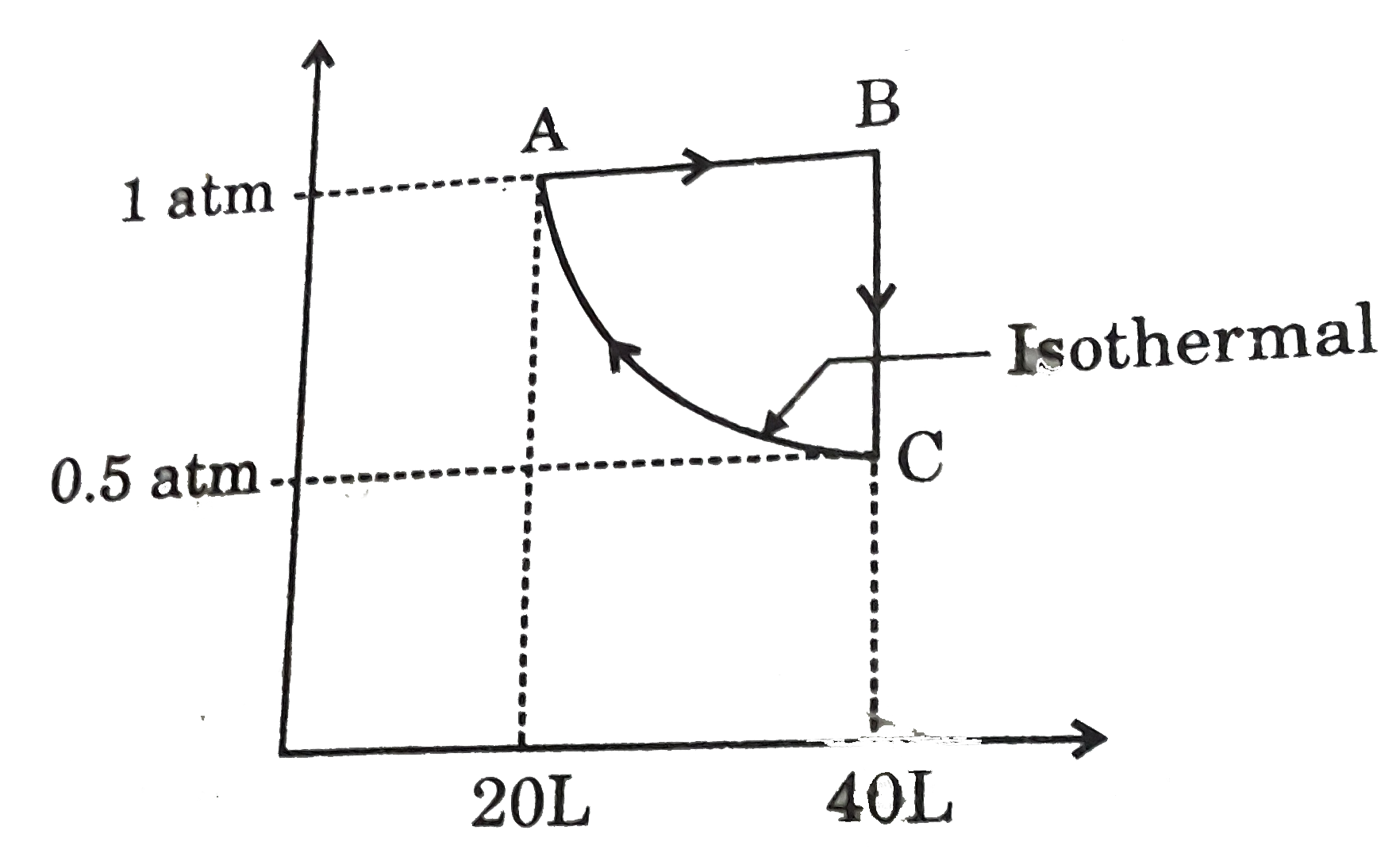
- An ideal gas in subjected to a three step reversible process as shown....
Text Solution
|
- A certain mass of an ideal gas absorbes 80 kJ heat and gas is expended...
Text Solution
|
- One mole of an ideal gas is undergoing process as shown in figure then...
Text Solution
|
- An ideal gas in subjected to a three step reversible process as shown....
Text Solution
|
- Calculate DeltaG (in L atm) from the graph of an ideal gas undergoing ...
Text Solution
|
- 2 moles of an ideal monoatomic gas undergoes a cyclic process ABCA as ...
Text Solution
|
- Three moles of an ideal gas expands reversibly under isothermal condit...
Text Solution
|
- A system consisting of 1 mol of an ideal gas undergoes a reversible pr...
Text Solution
|
- One mole of ideal monoatmic gas is carried throught the reversible cyc...
Text Solution
|