Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following pairs has higher resonance energy: and CH(2...
Text Solution
|
- The IUPAC name of CH(2) =CH-CH=CH-underset(CH=CH(2))underset(|)(CH)-C-...
Text Solution
|
- Which of the following pairs has higher resonance energy: (a). CH(3)...
Text Solution
|
- Which of the followig pairs has higher resonance energy: (a). (b)...
Text Solution
|
- Which compound has higher resonance energy in the following pairs. (C)...
Text Solution
|
- IUPAC name is CH(3)-CH=CH-CH=CH(2)
Text Solution
|
- CH(2)=CH-CH=CH-CH=CH(2)
Text Solution
|
- Draw the resonance structures of the following compounds. (a) CH(2) ...
Text Solution
|
- Draw the resonance structures of the following compounds : (i) CH(2) =...
Text Solution
|
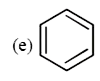 and `CH_(2) = CH - CH = CH - CH = CH_(2)`
and `CH_(2) = CH - CH = CH - CH = CH_(2)`