A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-NEET 2018-QUESTION
- Carboxylic acid have higher boiling points than aldehydes, ketones an...
Text Solution
|
- Compound A,C(8)H(10)O, is found to react with NaOI (produced by reacti...
Text Solution
|
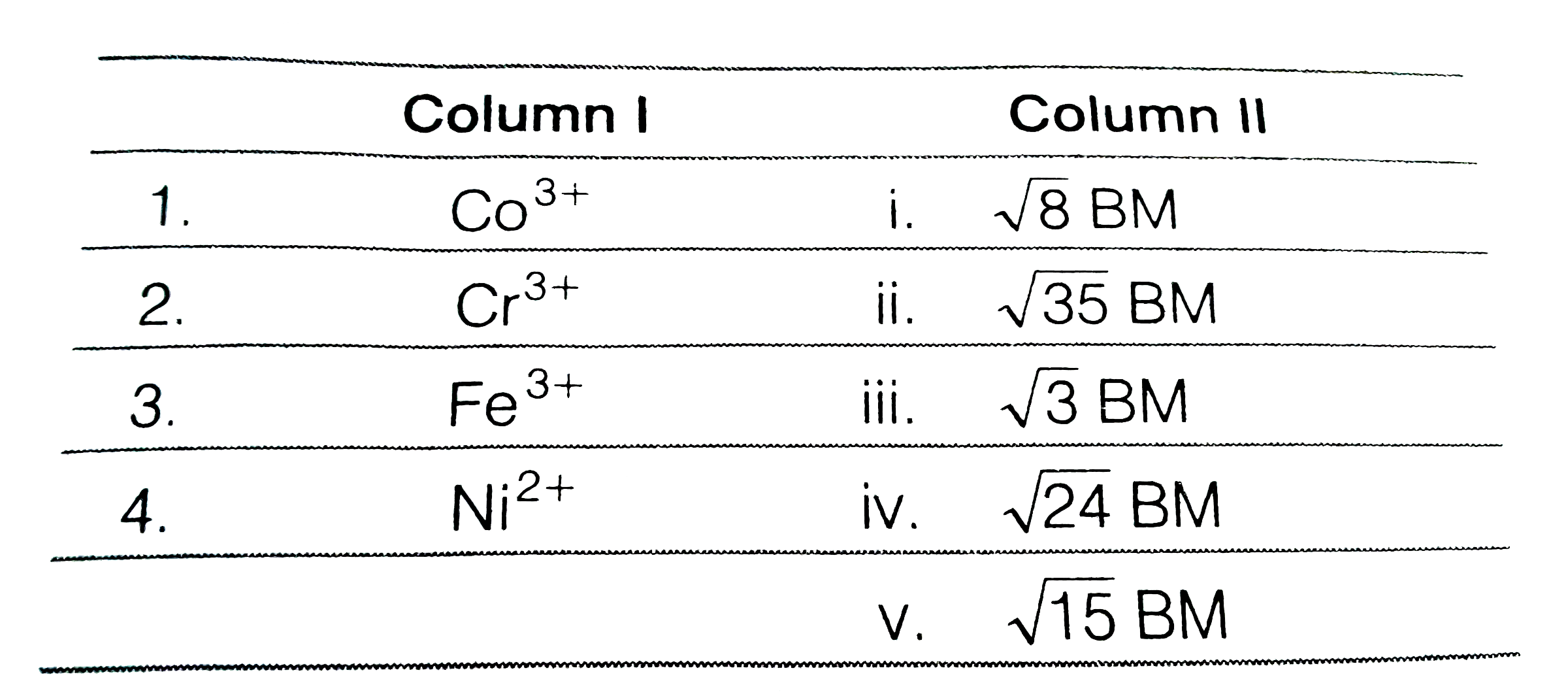
- Match the metal ions given in Column I with the spin magnetic moments ...
Text Solution
|
- Which one of the following ions exhibits d-d transition and paramagnet...
Text Solution
|
- Iron carbonyl, Fe(CO)5 is
Text Solution
|
- The type of isomerism shown by the complex [COCl(2)(en)(2)] is
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- Following solutions were prepared by mixing different volumes of NaOH ...
Text Solution
|
- On which of the following properties does the coagulating power of an ...
Text Solution
|
- Given van der Waals constant for NH(3), H(2), O(2) and CO(2) are respe...
Text Solution
|
- The solubility of BaSO(4) in water is 2.42xx10^(-3) gL^(-1) at 298 K. ...
Text Solution
|
- In which case is the number of molecules of water maximum?
Text Solution
|
- The correct difference between first and second order reactions is tha...
Text Solution
|
- Among CaH(2),BeH(2),BaH(2), the order of ionic character is
Text Solution
|
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- For the redox reaction, MnO(4)^(-) + C(2)O(4)^(2-) + H^(+) rarr Mn^(...
Text Solution
|
- Which one of the following condition will favour maximum formation of ...
Text Solution
|
- When initial concentration of the reactant is doubled, the half-life p...
Text Solution
|
- If the bond dissociation energies of XY,X(2) and Y(2) are in the ratio...
Text Solution
|
- The correction factor 'a' to the ideal gas equation corresponds to
Text Solution
|