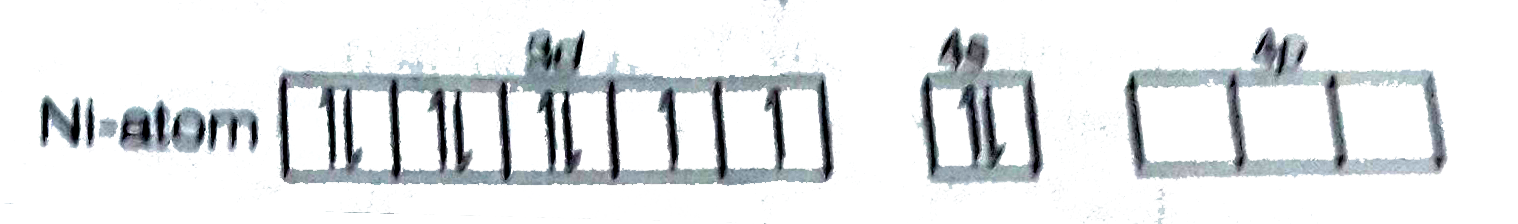A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
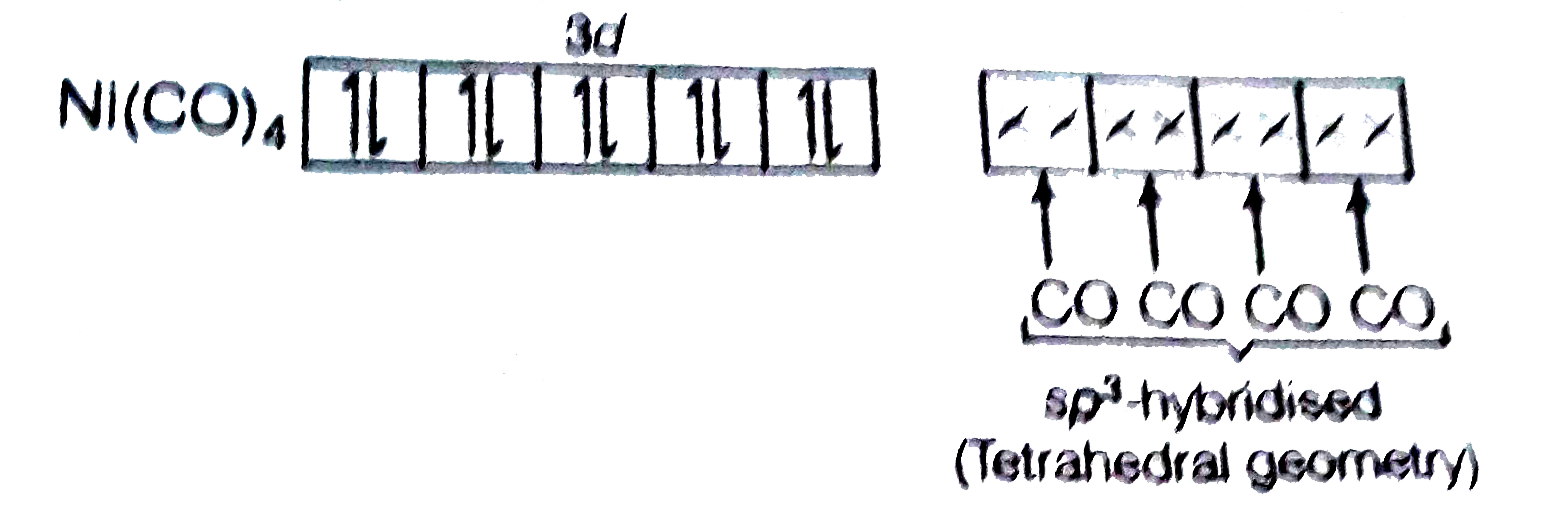
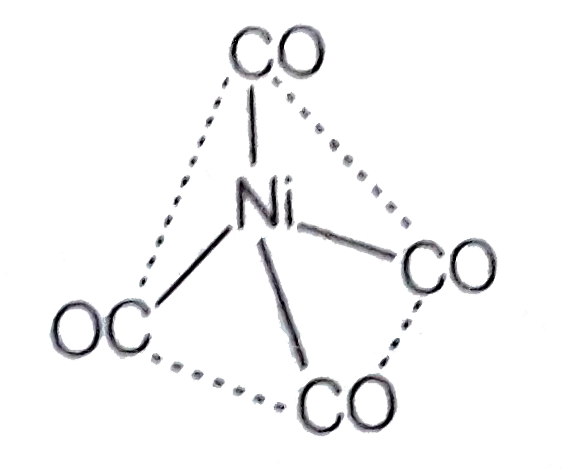
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- Explain how two complexes of nickel, [Ni(CN)4]^(2-) and Ni(CO)4 have d...
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)(4)] are -
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)(4)] are-
Text Solution
|
- The geometry and magnetic behavior of the complex [Ni(CO)4] are
Text Solution
|
- [Ni(CO)4]द्वारा ज्यामिति प्राप्त होती है
Text Solution
|
- The geometry of. Ni(CO)4 and Ni(PPH3)2CI2 are :
Text Solution
|
- The geometry of Ni(CO)4 and Ni(PPh3)2CI2 are :
Text Solution
|