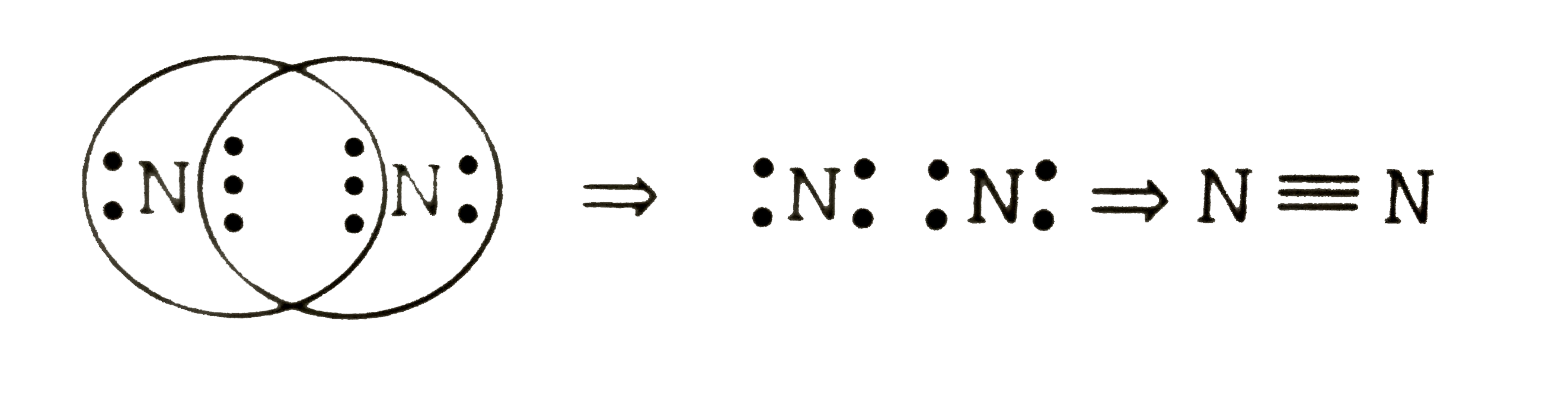A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-P-BLOCK ELEMENTS-Exercise
- Nitrogen is relatively inactive element because
Text Solution
|
- Cane sugar on reaction with nitric acid gives
Text Solution
|
- Number of electrons shared in the formation of nitrogen molecules i...
Text Solution
|
- Water gas is produced by
Text Solution
|
- Which of the following forces bind together carbon atoms in diamond?
Text Solution
|
- Which is used in the laboratory for last drying of neutral gases?
Text Solution
|
- the bleaching action of chlorine is due to
Text Solution
|
- Pure nitrogen is prepared in the laboratory by heating a mixture of
Text Solution
|
- PH(4)I+NaOH rarr? The product is
Text Solution
|
- PCl(3) reacts with water to form :
Text Solution
|
- Basicity of orthophosphoric acid is
Text Solution
|
- P(2)O(5) is heated with water to give
Text Solution
|
- Aqueous solution of ammonia consists of
Text Solution
|
- Oleum is
Text Solution
|
- Which would quickly absorb oxygen?
Text Solution
|
- Glass is a:
Text Solution
|
- Which of the following statement is not correct for nitrogen ?
Text Solution
|
- Bleaching powder reacts with a few drops of conc.HCl to yield
Text Solution
|
- it is possible to obtain oxygen from air by fractional distillation be...
Text Solution
|
- The gases respectively absorbed by alkaline pyrogallon and oil of cinn...
Text Solution
|