Similar Questions
Explore conceptually related problems
Recommended Questions
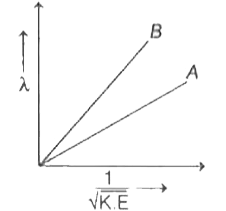
- The following plot represents the de-Broglie wavelength as a function ...
Text Solution
|
- De-Broglie wavelengths of the particle increases by 75% then kinetic e...
Text Solution
|
- For particles having same K.E., the de-Broglie wavelength is
Text Solution
|
- If the kinetic energy of a particle is doubled, de Broglie wavelength ...
Text Solution
|
- If the kinetic energy of a particle is doubled, De-Broglie wavelength ...
Text Solution
|
- K गतिज ऊर्जा वाले कर्ण की डी ब्रोग्ली तरंगदैर्ध्य lambda है यदि कण...
Text Solution
|
- The de Broglie wavelength (lambda) of a particle is related to its kin...
Text Solution
|
- The following plot represents the de-Broglie wavelength as a function ...
Text Solution
|
- Draw a plot showing the variation of de-Broglie wavelength of electron...
Text Solution
|