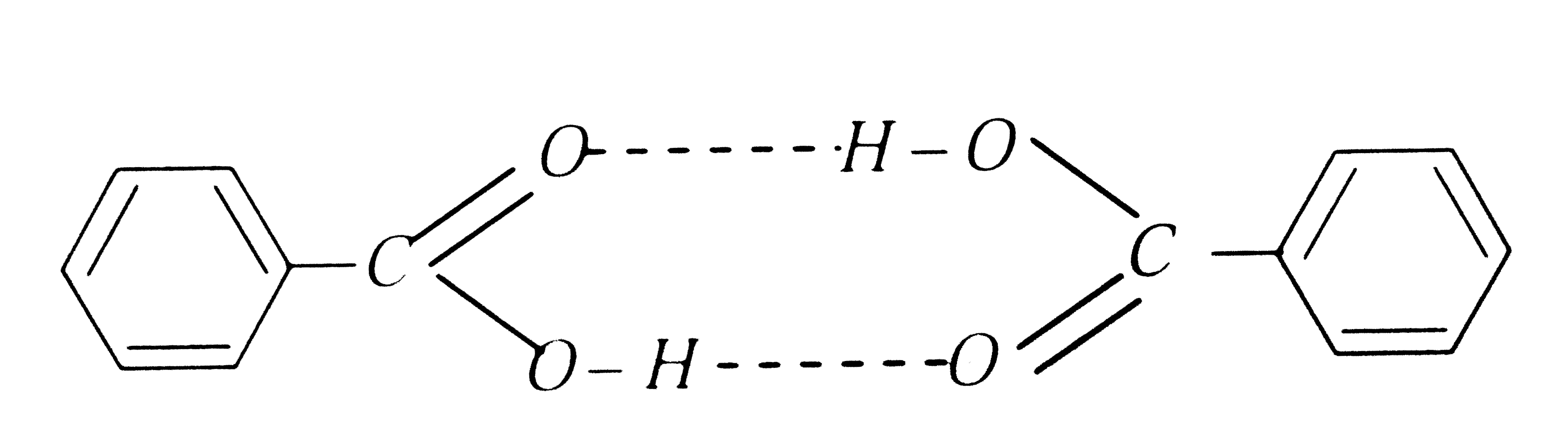A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTION
ERRORLESS |Exercise JEE SECTION (More than one choice correct answer)|13 VideosSOLUTION
ERRORLESS |Exercise JEE SECTION (Reasoning type questions)|2 VideosSOLUTION
ERRORLESS |Exercise Critical Thinking (Objective Questions)|28 VideosSOLID STATE
ERRORLESS |Exercise JEE section (Numeric )|1 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS |Exercise JEE Section (Matrix Match type questions)|3 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -SOLUTION -JEE SECTION (Only one choice correct answer)
- A 0.2 molal aqueous solution of weak acid (HX) is 20% ionised. The fre...
Text Solution
|
- A 0.1 molal aqueous solution of a weak acid is 30% ionized. If K(f) fo...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- The van't Hoff factor for 0.1 M Ba(NO(3))(2) solution is 2.74 . The de...
Text Solution
|
- The normality of 0.3 M phosphorous acid (H(3) PO(3)) is
Text Solution
|
- A 0.6% urea solution would be isotonic with :
Text Solution
|
- The molal freezing point constant of water is 1.86 K m^(-1). If 342 g...
Text Solution
|
- Which of the following solutions in water will have the lowest vapour ...
Text Solution
|
- During depression of freezing point in a solution, the following are i...
Text Solution
|
- The elevation in boiling point of a solution of 13.44g of CuCl(2)(mole...
Text Solution
|
- 75.2 g of C(6)H(5)OH (phenol) is dissolved in a solvent of K(f) = 14. ...
Text Solution
|
- When 20 g of naphthoic acid (C(11)H(8)O(2)) is dissolved in 50 g of be...
Text Solution
|
- The Henry's law constant fo the solubility of N(2) gas in water at 29...
Text Solution
|
- Dissolving 120g of urea (mol wt = 60) in 1000g of water gave a solutio...
Text Solution
|
- The freezing point (in .^(@)C) of a solution containing 0.1 g of K(3)[...
Text Solution
|
- For a dilute solution containing 2.5g of a non-volatile non-electrolyt...
Text Solution
|
- The molarity of a solution obtained by mixing 800 mL of 0.5 M HCl with...
Text Solution
|
- Consider separate solutions of 0.500 M C(2)H(5)OH(aq),0.100 M Mg(3)(PO...
Text Solution
|
- The vapour pressure of acetone at 20^(@)C is 185 torr. When 1.2 g of n...
Text Solution
|
- 18g of glucose (C(6)H(12)O(6))is added to 178.2 g of water. The vapour...
Text Solution
|