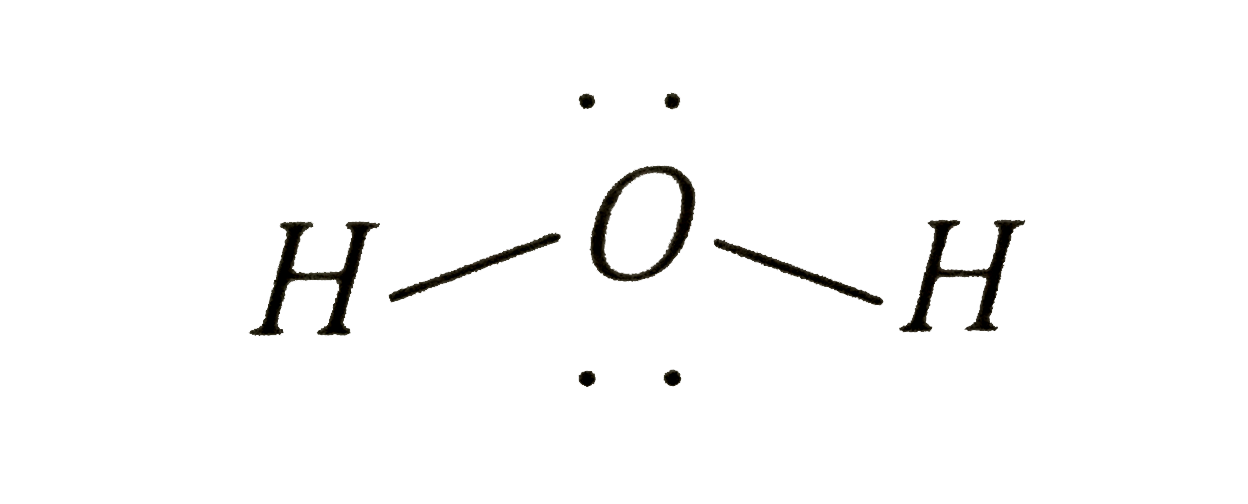
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
ERRORLESS |Exercise Ordinary thinking (Coordinate or dative bonding )|15 VideosCHEMICAL BONDING
ERRORLESS |Exercise Ordinary thinking (Dipole moment )|34 VideosCHEMICAL BONDING
ERRORLESS |Exercise JEE Section (Jee (advanced ) 2018)|1 VideosCARBOXYLIC ACID AND THEIR DERIVATIVES
ERRORLESS |Exercise Jee Section (Matrix Match type Questions)|2 VideosCHEMICAL EQULIBRIUM
ERRORLESS |Exercise JS JEE SECTION (ONLY ONE CHOICE ANSWER (Matrix)|1 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -CHEMICAL BONDING -Ordinary thinking (Covalent bonding)
- Among the following which property is commonly exhibited by a covalent...
Text Solution
|
- Which of the following is the correct electron-dot structure of N2O mo...
Text Solution
|
- How many electrons pairs are present in valence shell of oxygen in wat...
Text Solution
|
- Boron form covalent compound due to
Text Solution
|
- The following element forms a molecules with eight its own atoms
Text Solution
|
- Number of electrons in the valence orbit of nitrogen in an ammonia mol...
Text Solution
|
- Covalent compounds have low melting points because
Text Solution
|
- Sodium chloride is an ionic compound whereas hydrogen chloride is a ga...
Text Solution
|
- Which of the following is not the characteristic of a covalent compoun...
Text Solution
|
- Among the following groupings, which one represents the set of iso-ele...
Text Solution
|
- Among CaH2, NH3, NaH and B2H6 which are covalent hydrides?
Text Solution
|
- Among the following the maximum covalent character is shown by the com...
Text Solution
|
- Which of the following substances when dissolved in water will give a ...
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- If a molecule X(2) has a triple bond, then X will have the electronic ...
Text Solution
|
- Which of the following compounds does not follow the octet rule the el...
Text Solution
|
- Why is H(2)S more acidic than water ?
Text Solution
|
- The electronic configuration of four elements L, P, Q and R are given ...
Text Solution
|
- Which of the following occurs, when two hydrogen atoms bond with each ...
Text Solution
|
- Indicate the nature of bonding in C Cl(4) and CaH(2)
Text Solution
|