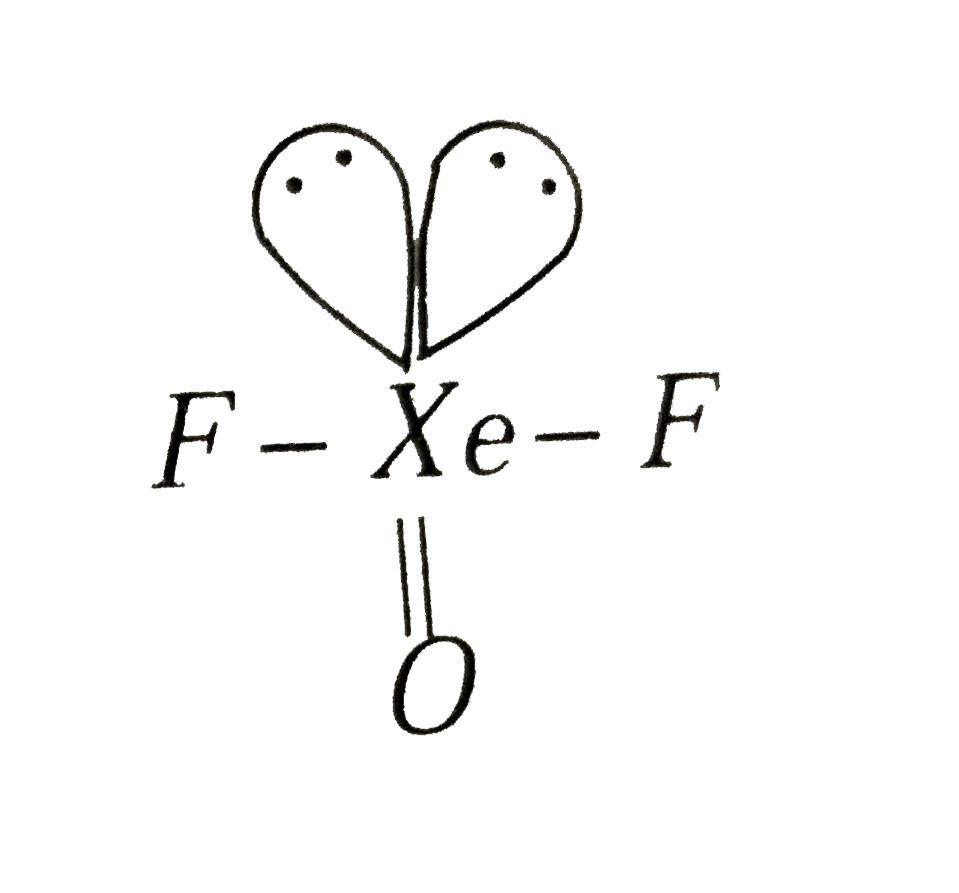A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The shape of "XeOF"(2) on the basis of VSEPR theory is
Text Solution
|
- The shape of "XeOF"(2) on the basis of VSEPR theory is
Text Solution
|
- Discuss the molecular shape of "BrF"(3) on the basis of VSEPR theory.
Text Solution
|
- Explain the shape of XeF(4) on the basis of VSEPR theory.
Text Solution
|
- On the basis of VSEPR theory predict the shape of the Ozone .
Text Solution
|
- Discuss the molecular shape of BrF3 on the basis of VSEPR theory.
Text Solution
|
- Discuss the shapes of XeO3 and XeOF4 on the basis of VSEPR theory.
Text Solution
|
- On the basis of VSEPR theory explain the shape of BrF3 molecule .
Text Solution
|
- Predict the shape on the basis of VSEPR theory. BeCl2
Text Solution
|