A
B
C
D
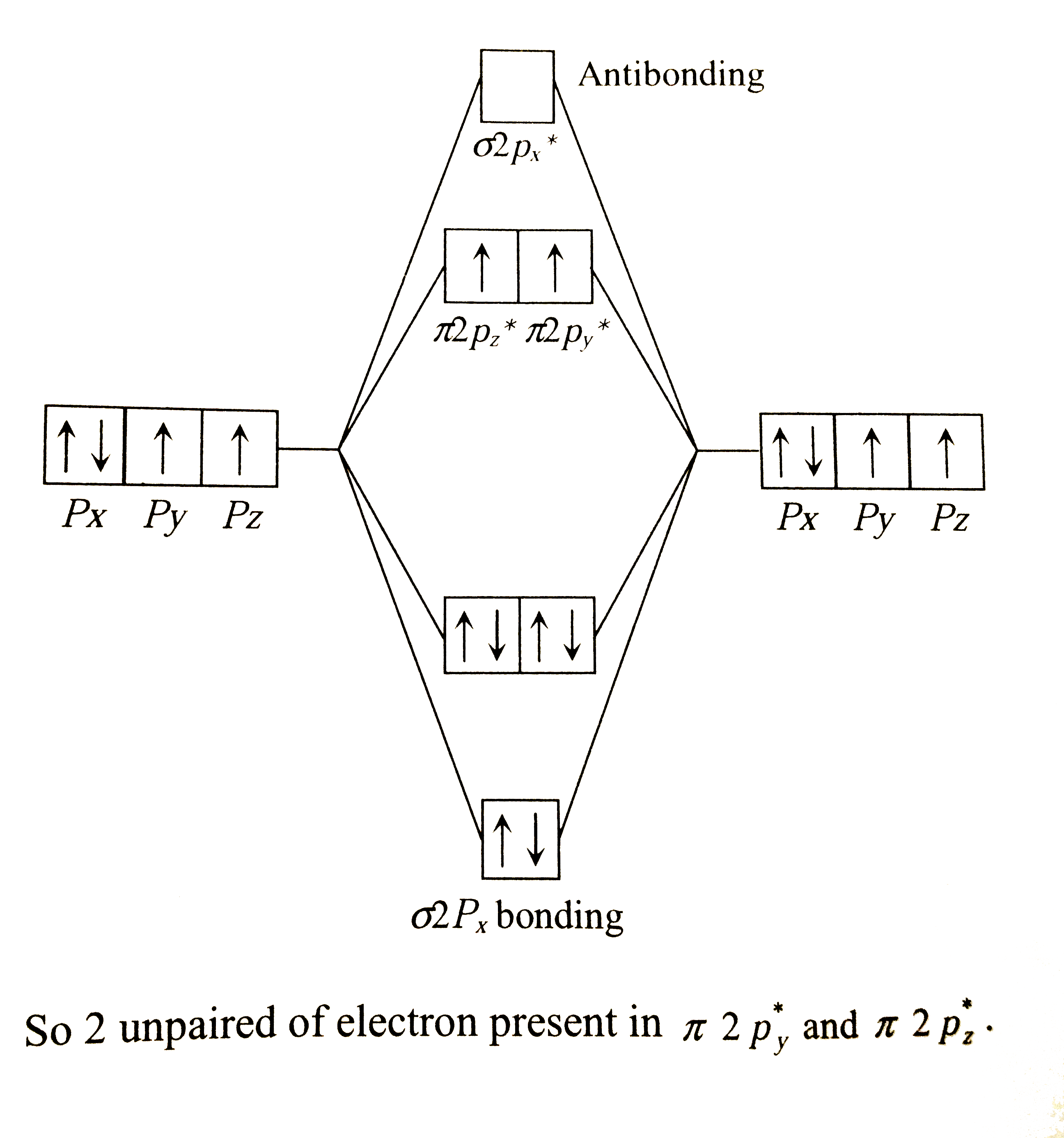
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The paramagnetic property of the oxygen molecule is due to the presenc...
Text Solution
|
- Which of the following molecules has one unpiared electron in antibond...
Text Solution
|
- The paramagnetic property of the oxygen molecule is due to the presenc...
Text Solution
|
- The compound which is coloured and paramagnetic due to the presence of...
Text Solution
|
- Oxygen molecule is paramagnetic because
Text Solution
|
- The paramagnetism of O(2)^(+) is due to the presence of an odd electro...
Text Solution
|
- According to molecular orbital theory, the paramagnetism of O(2) molec...
Text Solution
|
- Paramagnetism is due to the presence of electrons .
Text Solution
|
- The paramagnetic character of transition metals is due to the presence...
Text Solution
|