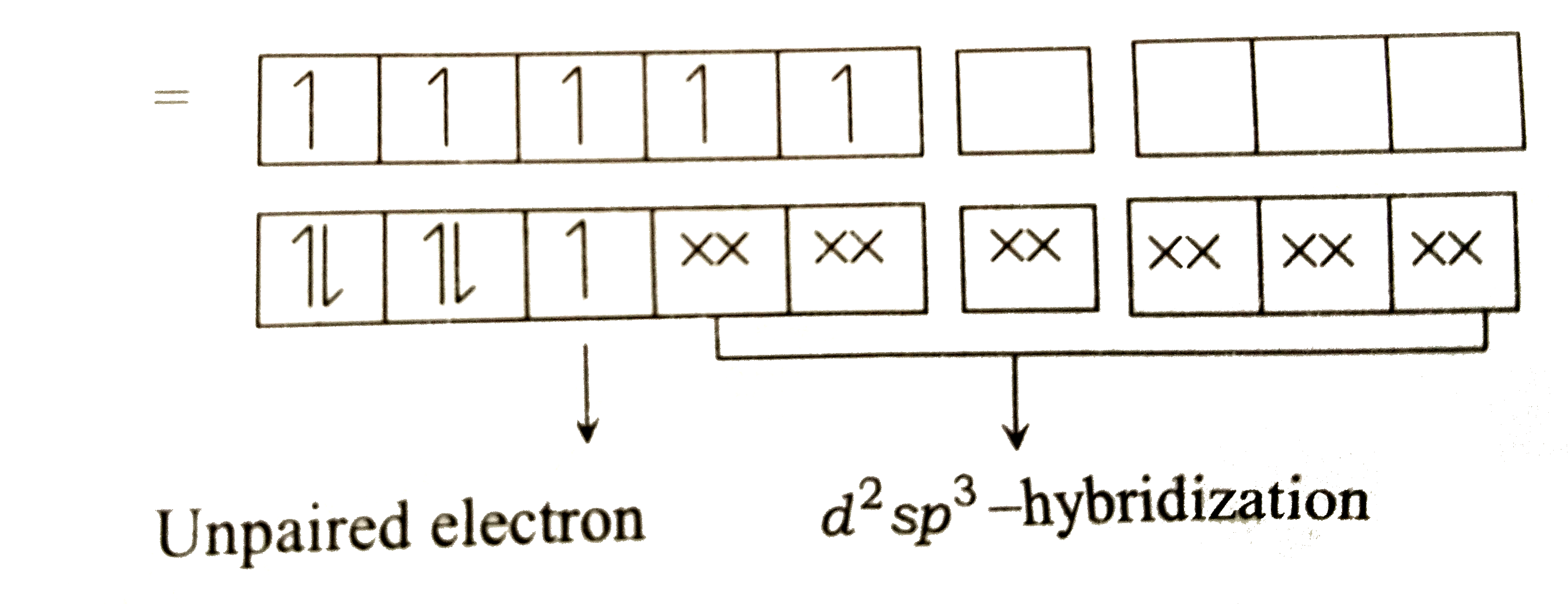
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
ERRORLESS |Exercise JEE Section (Only one choice correct answer)|80 VideosCHEMICAL BONDING
ERRORLESS |Exercise JEE Section (More than one correct answer)|12 VideosCHEMICAL BONDING
ERRORLESS |Exercise Ordinary thinking (Types of bonding and force in solid )|19 VideosCARBOXYLIC ACID AND THEIR DERIVATIVES
ERRORLESS |Exercise Jee Section (Matrix Match type Questions)|2 VideosCHEMICAL EQULIBRIUM
ERRORLESS |Exercise JS JEE SECTION (ONLY ONE CHOICE ANSWER (Matrix)|1 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -CHEMICAL BONDING -Critical thinking( Objective question)
- Which of the following species contains three bond pairs and one lone ...
Text Solution
|
- Which of the following has the highest dipole moment ?
Text Solution
|
- Which of the following arrangment of molecules is correct on the basi...
Text Solution
|
- Sulphur reacts with chlorine in 1:2 ration and forms X . Hydrolysis of...
Text Solution
|
- Bond length of ethane (I), ethene (II), acetylene (III) and benzene (I...
Text Solution
|
- Match the list I and list II and write the correct matching
Text Solution
|
- O(2)^(2-) is the symbol of ...... Ion
Text Solution
|
- The values of electronegativity of atom A and B are 1.20 and 4.0 respe...
Text Solution
|
- Fluorine reacts with dilute NaOH and forms a gaseous product A. The bo...
Text Solution
|
- Hybridisation state of chlorine in ClF(3) is
Text Solution
|
- Among the following , the compound that contains ionic, covalent and c...
Text Solution
|
- Which one of the following contains ionic, covalent and co-ordinate an...
Text Solution
|
- The electronegativity of an element is low. The bond formed between tw...
Text Solution
|
- The magnetic moment of K(3)[Fe(CN)(6)] is found to be 1.7 B.M. how man...
Text Solution
|
- It is believed that atoms combine with each other such that the outerm...
Text Solution
|
- Which of the following contains a coordinate and covalent bond
Text Solution
|
- Structure of IF(4)^(+) and hybridization of iodine in this structure a...
Text Solution
|
- In which of the following the central atoms does not use sp^(3) hybrid...
Text Solution
|
- The ionisation of hydrogen atom would give rise to
Text Solution
|
- Which can described as a molecule with residual bonding capacity
Text Solution
|