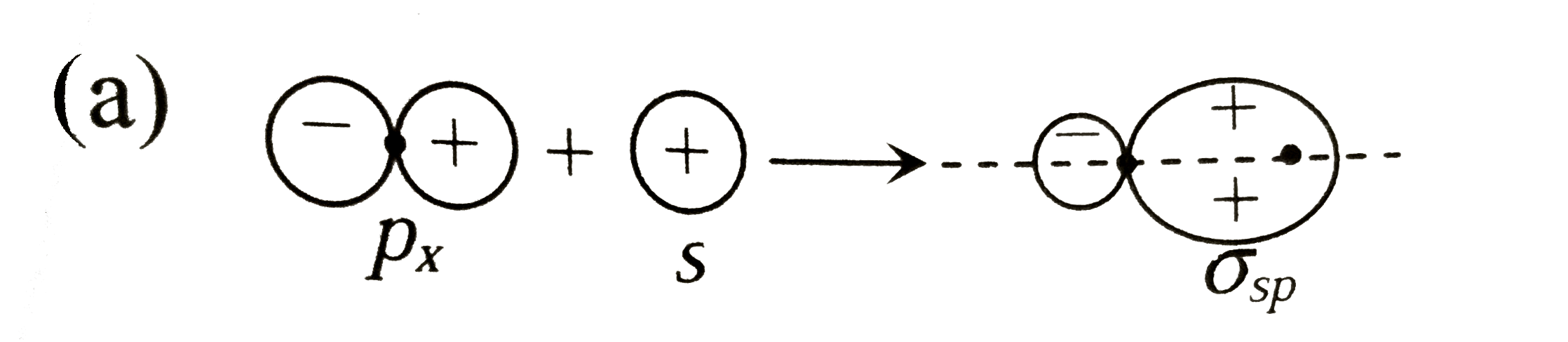A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|