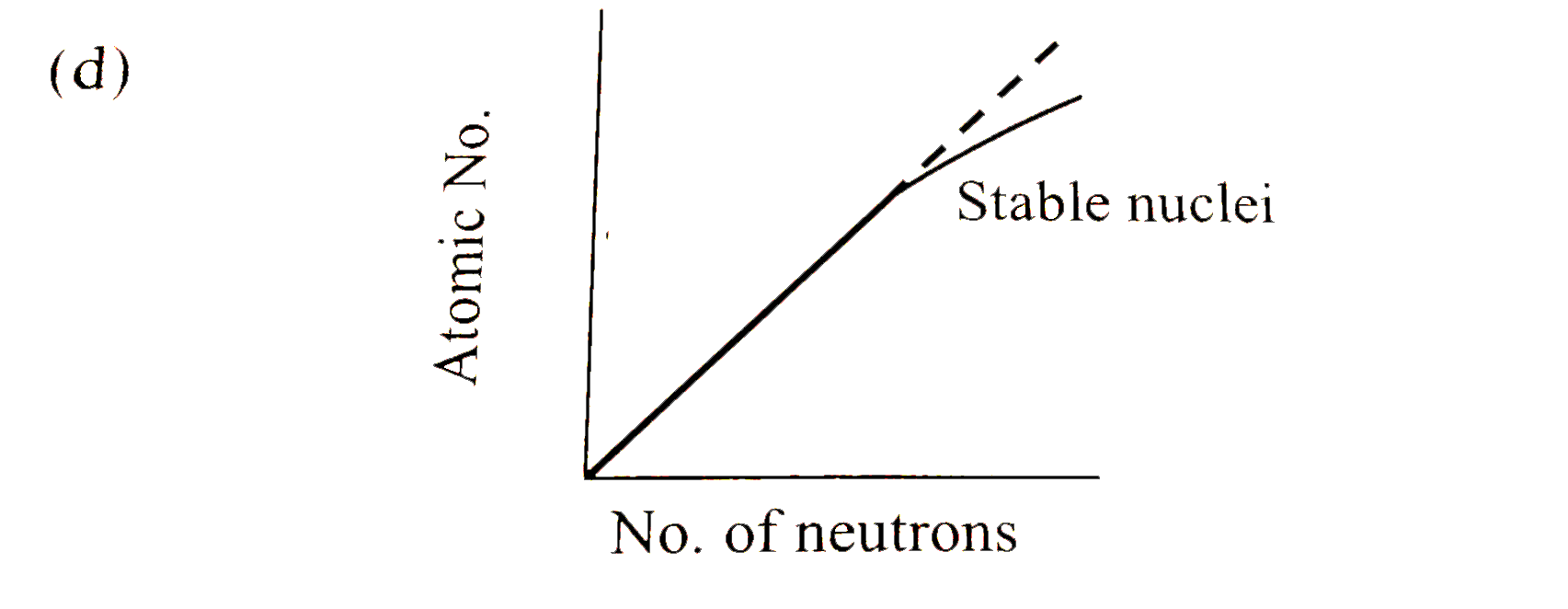A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Statement 1 : Proton -proton electrostatic repulsions begin to overcom...
Text Solution
|
- Statement : The plot of atomic number ( y -axis ) versus number of neu...
Text Solution
|
- Statement : The plot of atomic number ( y -axis ) versus number of neu...
Text Solution
|
- Statement 1 : Proton -proton electrostatic repulsions begin to overcom...
Text Solution
|
- Statement-1:Light nuclei having equal number of protons and neutrons a...
Text Solution
|
- Statement-1: Th plot of atomic number (y-axis) versus number of neutro...
Text Solution
|
- कथन : स्थायी नाभिक के लिए परमाणु क्रमांक (अक्ष) तथा न्यूट्रॉनों की संख...
Text Solution
|
- The sum of the proton and neutron numbers in an atom?
Text Solution
|
- कथन : स्थायी नाभिक के लिए परमाणु क्रमांक (अक्ष) तथा न्यूट्रॉनों की संख...
Text Solution
|