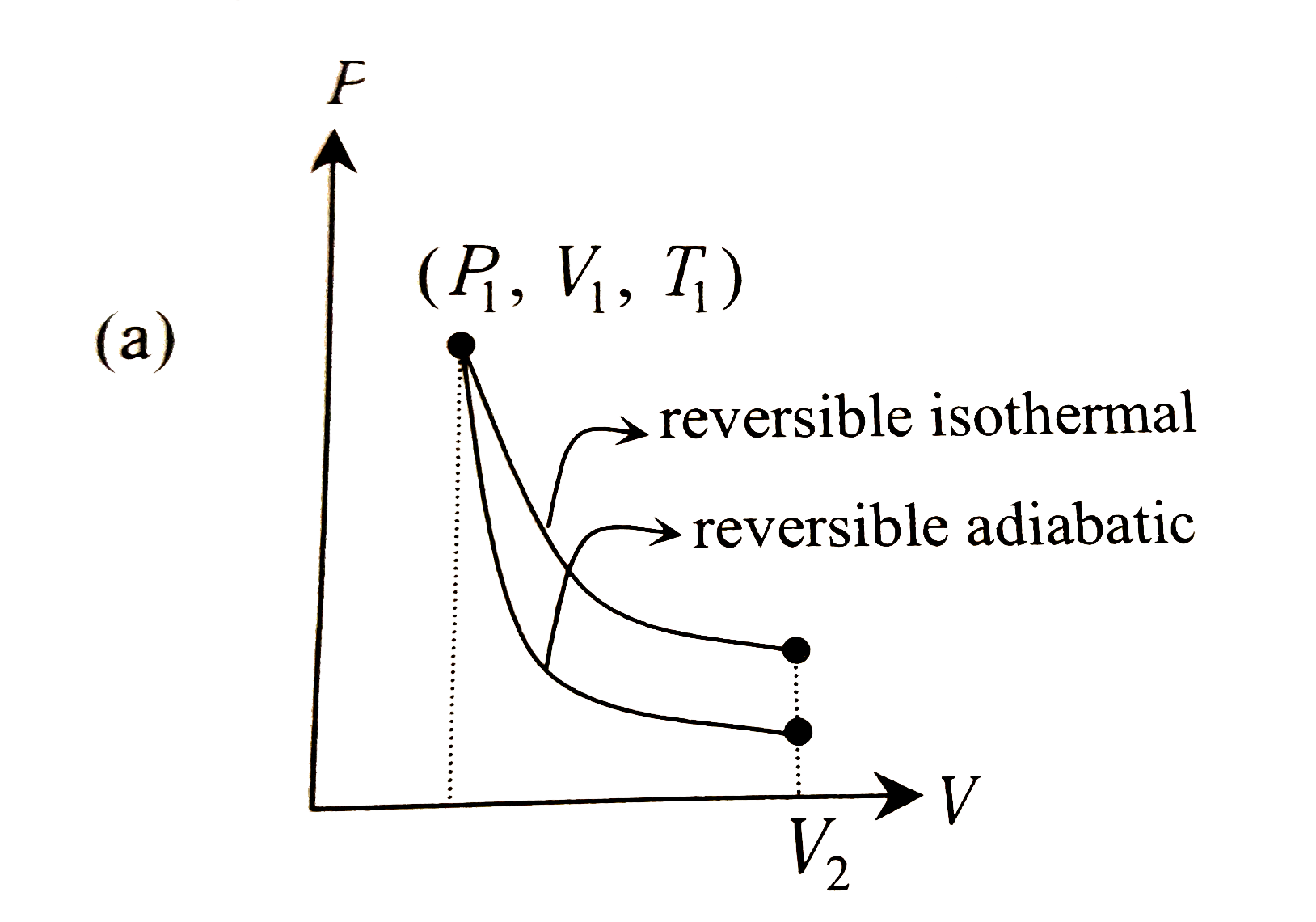A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS |Exercise JEE section (Reasoning type questions)|6 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS |Exercise JEE section (Comprehension type questions)|9 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS |Exercise JEE section (Only one correct answer)|42 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS |Exercise JEE Section (Matrix Match type questions)|3 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -THERMODYNAMICS AND THERMOCHEMISTRY -JEE section (More than one choice correct answer)
- The following is (are) endothermic reaction (s)
Text Solution
|
- Identify the intensive quantities from the following :
Text Solution
|
- The enthalpy of neutralization of which of the following acid & base i...
Text Solution
|
- At 300 K, the reaction with which of the following values of thermodyn...
Text Solution
|
- Which one of the following statement is false
Text Solution
|
- Among the following , the state funcation (s) is (are)
Text Solution
|
- Among the following, the intensive property is (properties are):
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- An ideal gas in a thermally insulated vessel at internal pressure = P(...
Text Solution
|
- An ideal gas is expand from (p(1),V(1),T(1)) to (p(2),V(2),T(2)) under...
Text Solution
|
- For a reaction taking place in a container in equilibrium with its sur...
Text Solution
|
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- If separate samples of argon, methane, nitrogen and ammonia, all at th...
Text Solution
|
- Which of the following is/are correct
Text Solution
|