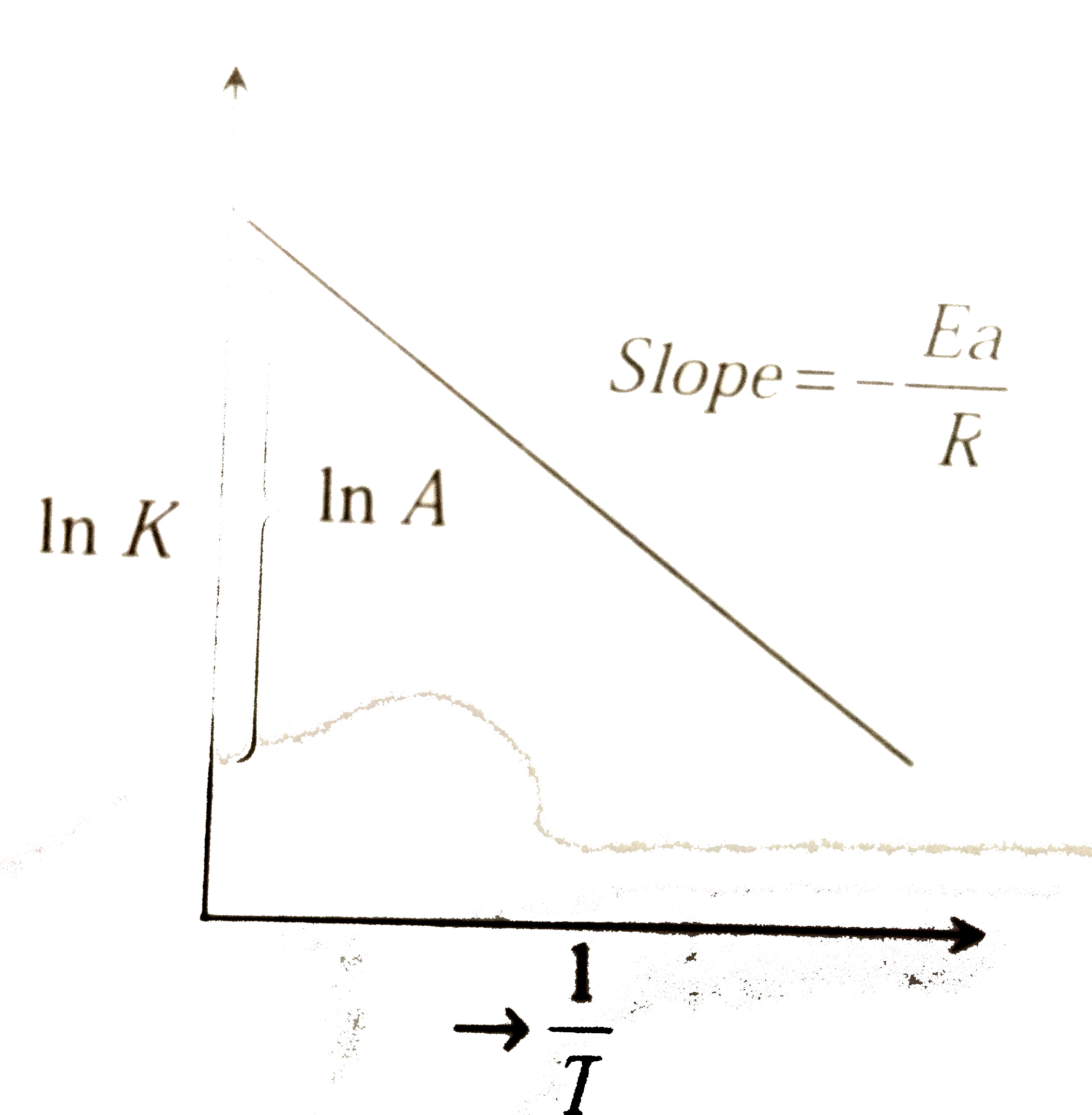A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS |Exercise Critical Thinking Objective Questions|20 VideosCHEMICAL KINETICS
ERRORLESS |Exercise JEE SECTION (Only one choice correct answer)|23 VideosCHEMICAL KINETICS
ERRORLESS |Exercise Ordinary Thinking (Rate law and Rate constant )|189 VideosCHEMICAL EQULIBRIUM
ERRORLESS |Exercise JS JEE SECTION (ONLY ONE CHOICE ANSWER (Matrix)|1 VideosCHEMISTRY IN EVERYDAY IN LIFE
ERRORLESS |Exercise Critical Thinking (Objective question )|25 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -CHEMICAL KINETICS -Ordinary Thinking (Collision theory , Energy of activation and Arrhenius equation )
- For an endothermic reaction, energy of activation is Ea and enthalpy o...
Text Solution
|
- Activation energy (E(a)) and rate constants (k(1) and k(2)) of a chemi...
Text Solution
|
- The activation energy of a reaction can be determined from the slope o...
Text Solution
|
- According to law of photochemical equivalence the energy absorbed (in ...
Text Solution
|
- An endothermic reaction with high activation energy for the forward re...
Text Solution
|
- Consider an endothermic reaction XrarrY with the activation energies E...
Text Solution
|
- According to the adsorption theory of catalysis, the speed of the reac...
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k = Ae...
Text Solution
|
- For a reaction taking place in three steps, the rate consatnt are k(1)...
Text Solution
|
- If the activation enery for the forward reaction is 150 "kJ mol"^(-1) ...
Text Solution
|
- In the Arrhenius plot of ln k vs (1)/(T) , a linear plot is obtained w...
Text Solution
|
- If the reaction rate at a given temperature becomes slower
Text Solution
|
- A large increase in the rate of a reaction for a rise in temperature i...
Text Solution
|
- The minimum energy required for molecules to enter into the reaction i...
Text Solution
|
- The rate constant of a reaction at temperature 200 K is 10 times less ...
Text Solution
|
- On increasing the temperature, the rate of the reaction increases beca...
Text Solution
|
- A reaction having equal energies of activation for forward and reverse...
Text Solution
|
- A reaction rate constant is given by : K= 1.2 xx10^(14)e^((-25000)/(RT...
Text Solution
|
- The Arrhenius equation expressing the effect of temperature on the rat...
Text Solution
|
- The rate constant is given by the equation k = P.Ze^(-E//RT). Which fa...
Text Solution
|