A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
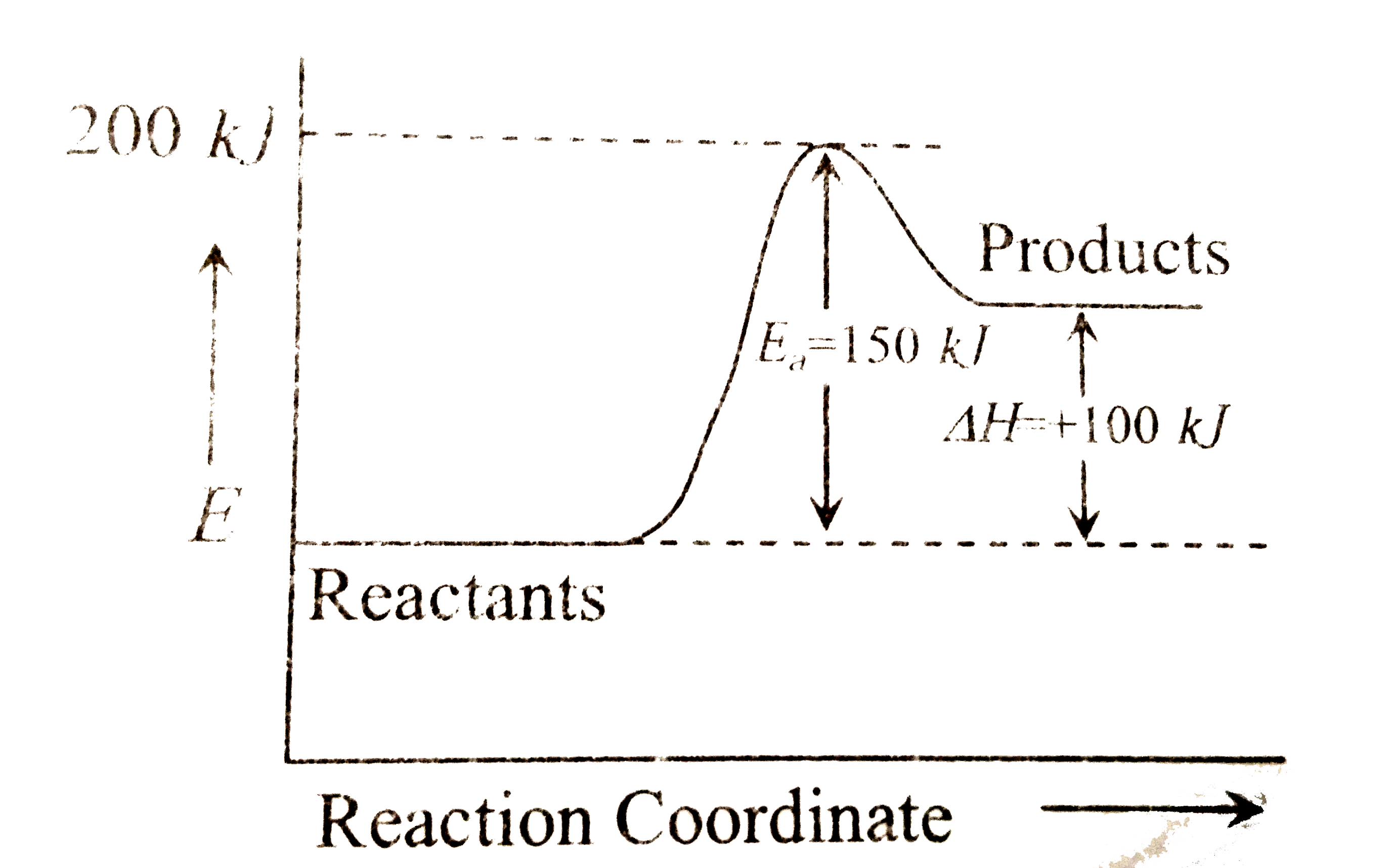
- In the given graph the activation energy , E(a) for the reverse reacti...
Text Solution
|
- If the activation energies of the forward and backward reactions of a ...
Text Solution
|
- The activation energy for a ismple chemical reaction A rarr B is E(a) ...
Text Solution
|
- Energy of activation for a reversible reaction is 6 kcal (E(a) forward...
Text Solution
|
- in a reversible reaction the energy of activation of the forward react...
Text Solution
|
- The activation energy for a simple chemical reaction A rarr B is E(a) ...
Text Solution
|
- In the given graph the activation energy , E(a) for the reverse reacti...
Text Solution
|
- उच्च सक्रियण ऊर्जा वाली अभिक्रियाएँ मन्दगामी अभिक्रियाएँ होती है।
Text Solution
|
- यदि अग्रिम तथा प्रतीप अभिक्रियाओं की सक्रियण ऊर्जाएँ क्रमशः E(f) तथा E...
Text Solution
|