Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
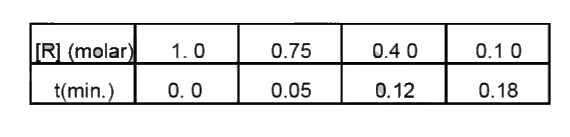
- The concentration of R in the reaction Rrarr P was measured as a funct...
Text Solution
|
- The concentration of R in the reaction R rarr P was measured as a func...
Text Solution
|
- The concentration of R in the reaction R to P was measured as a funcat...
Text Solution
|
- For the reaction R rarr P when concentration of R is made double the r...
Text Solution
|
- The concentration R in the reaction RtoP was measured as a function of...
Text Solution
|
- In reaction, ArarrB +C the following data were obtained. {:("t in seco...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measu...
Text Solution
|
- The concentration of R in the reaction R rarr P was measured as a func...
Text Solution
|
- The concentration of R in the reaction Rrarr P was measured as a funct...
Text Solution
|