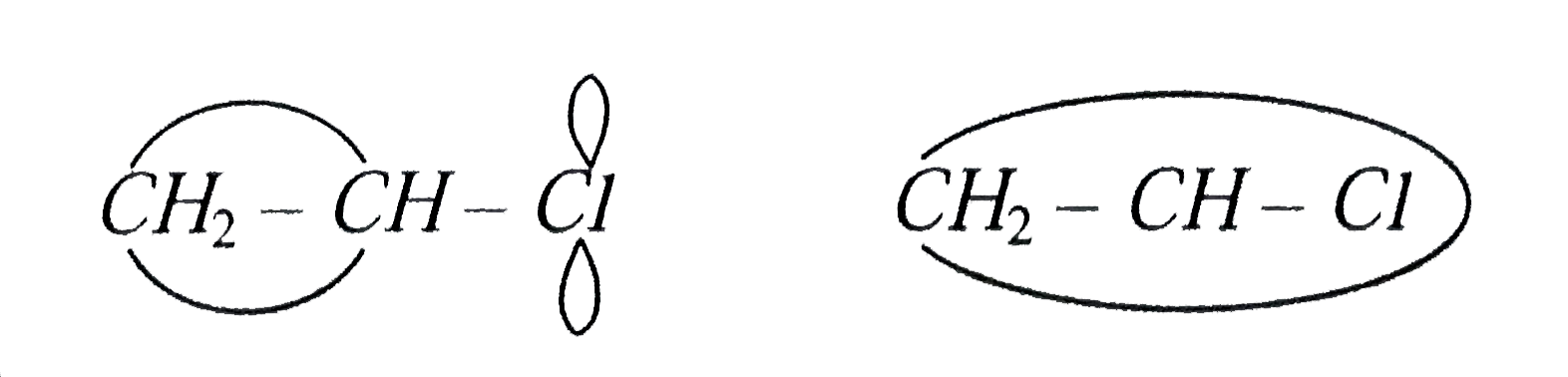A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is lest reactive in a nucleophilic .
Text Solution
|
- Which of the following is lest reactive in a nucleophilic .
Text Solution
|
- Which of the following alkoxides is the most reactive nucleophile?
Text Solution
|
- Which of the following is least reactive in a nucleophilic substiution...
Text Solution
|
- Which of the following is the least reactive towards nucleophile?
Text Solution
|
- Which of the following is least reactive in a nucleophilic substitutio...
Text Solution
|
- Which of the following is lest reactive in a nucleophilic .
Text Solution
|
- Which of the following is more reactive in nucleophilic substitution r...
Text Solution
|
- Which among the following is most reactive in nucleophilic addition ?
Text Solution
|