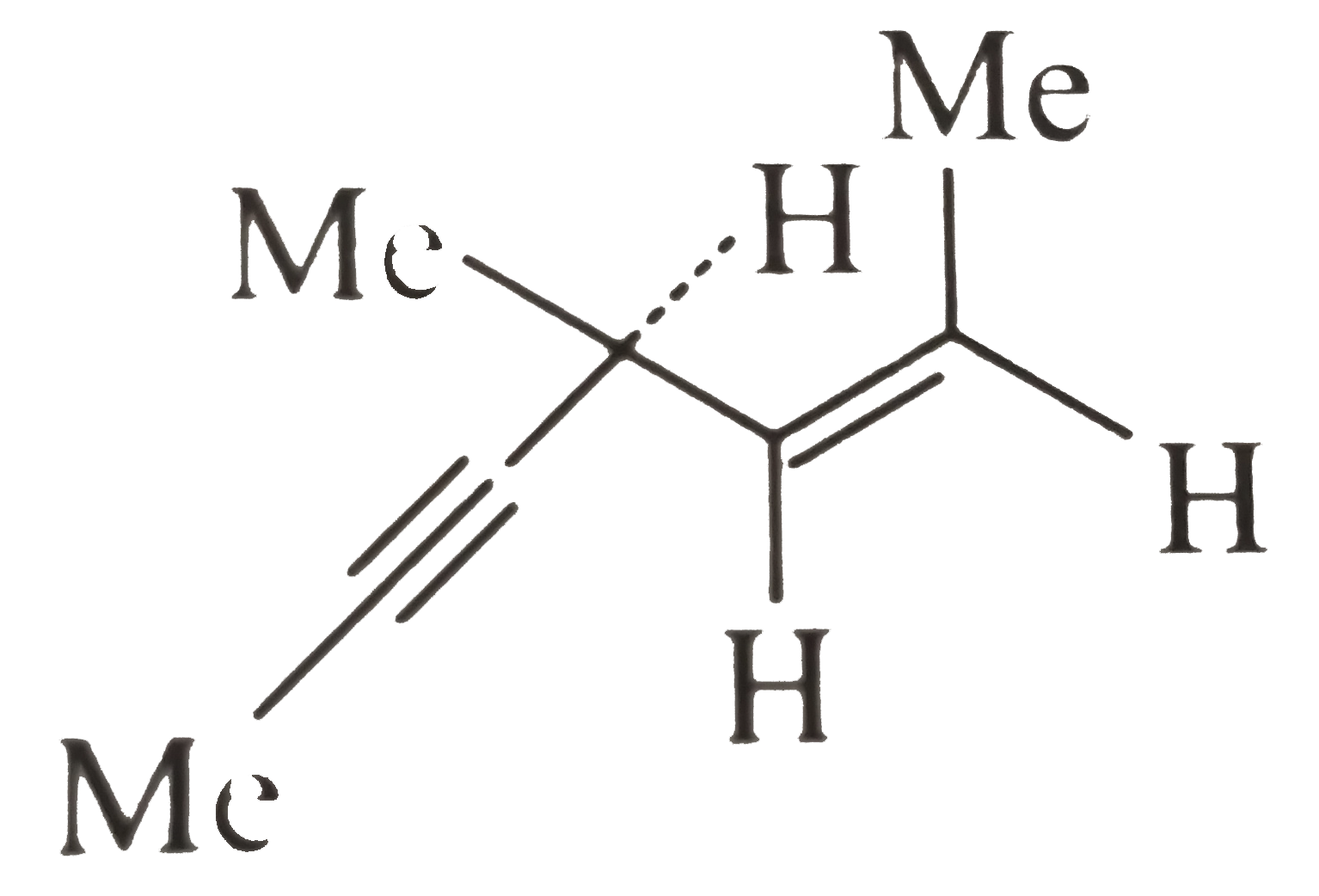
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise JEE Section (More than one choice correct answer)|32 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise Reasoning type questions|5 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS |Exercise Critical Thinking (Objective Questions)|44 VideosGASEOUS STATE
ERRORLESS |Exercise JEE SECTION JEE (Advanced) 2018 Numeric answer type question|1 VideosHALOGEN CONTAINING COMPOUND
ERRORLESS |Exercise Critical Thinking (Jee (Advanced) 2018)|1 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -GENERAL ORGANIC CHEMISTRY-JEE Section (Only one choice correct answer)
- As S(N^(2)) reaction at an asymmetric carbon of a compound always give...
Text Solution
|
- The number of isomers for the compound with molecular formula C(2)BrCl...
Text Solution
|
- Hydrogenation of the above compound in the presence of poisoned Pd cat...
Text Solution
|
- Identify the correct order of reactivity in electrophilic substitution...
Text Solution
|
- Which of the following compounds exhibits stereoisomerism?
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment
Text Solution
|
- Which of the following represent the given mode of hybridisation sp^(2...
Text Solution
|
- How many structures of F is possible?
Text Solution
|
- An enantiomerically pure acid is treated with racemic mixture of an al...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- On monochlorination of 2-methylbutane, the total number of chiral comp...
Text Solution
|
- The major product obtained when (Br2)/(Fe) is treated with
Text Solution
|
- C(2) is rotated anti-clockwise 120^(@) about C(2)-C(3) bond. The resul...
Text Solution
|
- Arrange in order of increasing acidic strength
Text Solution
|
- Which of the following resonating structures of 1-methoxy-1,3-butadien...
Text Solution
|
- C(5)H(11)Cloverset("frictional distillation")rarr M (isomeric product)...
Text Solution
|
- The IUPAC name of C(6)H(5)COCl is
Text Solution
|
- Among the following, the least stable resonance structure is :
Text Solution
|
- The number of stereoisomers obtained by bromination of trans-2-butene ...
Text Solution
|
- The correct statements (s) about the compound given below is/are:
Text Solution
|