A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
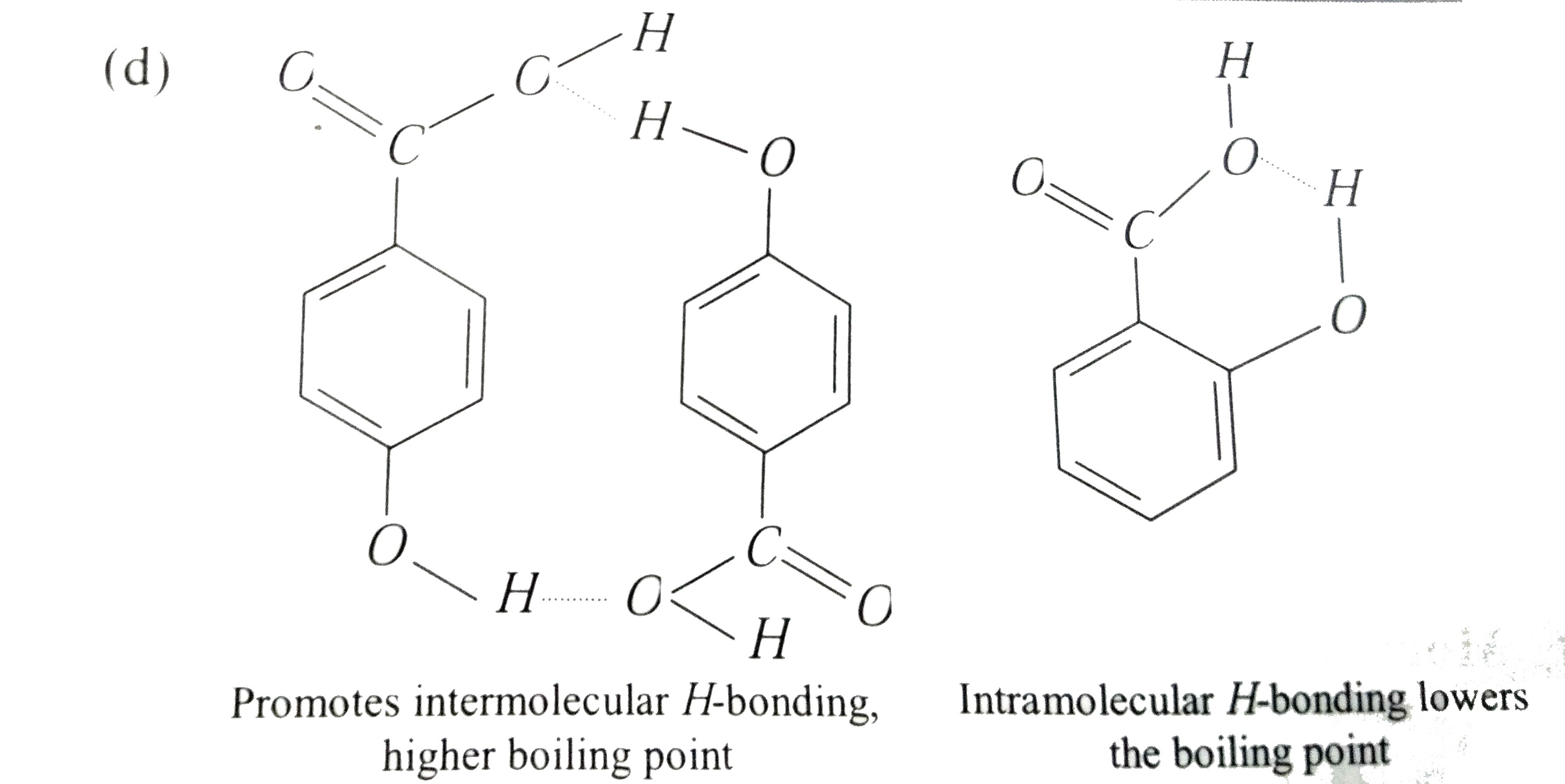
- Statement-1: p-Hydroxybenzoic acid has a lower boiling point then o-hy...
Text Solution
|
- Statement I : p-Hydroxybenzoic acid has a lower boiling point than o-h...
Text Solution
|
- Assertion: p- hydroxybenzoic acid has a lower boiling point than o- hy...
Text Solution
|
- Which of the following analgesics is an acetylation product of o-hydro...
Text Solution
|
- m-Hydroxybenzoic acid is a stronger acid than benzoic acid while p-hyd...
Text Solution
|
- Statement-1:p-Hydroxybenzoic acid has a lower boiling point that o-Hyd...
Text Solution
|
- Statement-I: p-Hydroxybenzoic acid has a lower boiling point that o-hy...
Text Solution
|
- Salicylic acid is a stronger acid than p-hydroxybenzoic acid due to
Text Solution
|
- Statement 1: p-hydroxybenzoic acid has a lower boiling point than o-hy...
Text Solution
|
 .
.