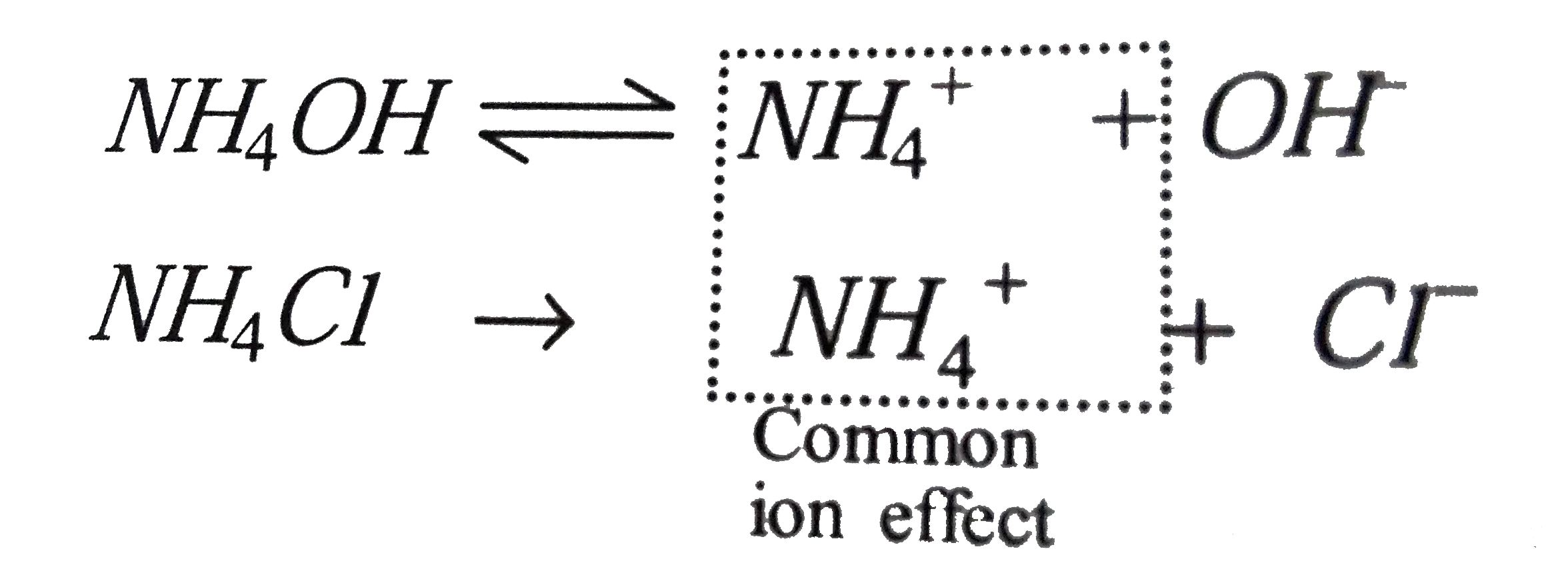
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ANALYTICAL CHEMISTRY
ERRORLESS |Exercise Ordinary Thinking Objective Questions (Volumetric Analysis)|72 VideosANALYTICAL CHEMISTRY
ERRORLESS |Exercise Critical Thinking Objective Questions|20 VideosANALYTICAL CHEMISTRY
ERRORLESS |Exercise Ordinary Thinking Objective Questions (Wet Test for Acid Radical)|26 VideosALDEHYDES AND KETONES
ERRORLESS |Exercise JEE Section (JEE (Advanced) 2018) Numeric answer type question|1 VideosBIOMOLECULES AND POLYMER
ERRORLESS |Exercise Jee section (Jee (Advanced 2018)) (More than one choice correct answer)|1 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -ANALYTICAL CHEMISTRY-Ordinary Thinking Objective Questions (Wet Test for Basic Radical)
- Ferric ion forms a prussian blue coloured ppt. due to
Text Solution
|
- When H(2)S gas is passed into a certain solution, it reacts to form a ...
Text Solution
|
- In the precipitation of the iron group in qualitative analysis, ammoni...
Text Solution
|
- Which one of the following can be used in place of NH(4)Cl for the ide...
Text Solution
|
- Sodium carbonate cannot be used in place of ammonium carbonate for the...
Text Solution
|
- Sometimes yellow turbidity appears while passing H(2)S gas even in the...
Text Solution
|
- Cu^(2+) ions will be reduced to Cu^(+) ions by the addition of an aque...
Text Solution
|
- Nessler's reagent is used to detect
Text Solution
|
- Which of the following on reaction with H(2)S does not produce metalli...
Text Solution
|
- With K(4)[Fe(CN)(6)],Cu^(2+) ion gives
Text Solution
|
- When H(2)S gas is passed thorugh the HCl containing aqueous solution o...
Text Solution
|
- A light greenish coloured salt was soluble in water ion passing H(2)S ...
Text Solution
|
- Which mixture is separated by conc. Aqueous solution of sodium hydroxi...
Text Solution
|
- Yellow ammonium sulphide solution is a suitable reagent for the separa...
Text Solution
|
- H(2)S is passed through as acidified solution of Ag, Cu and Zn. Which ...
Text Solution
|
- When dilute aqueous solution of AgNO3 (excess) is added to KI solution...
Text Solution
|
- A 0.3 M HCl solution contains the following ions Hg^(++),Cd^(++),Sr^(+...
Text Solution
|
- A compound is soluble in water. If ammonia is added, a red precipitate...
Text Solution
|
- A precipitate of the following would be obtained when HCl is added to ...
Text Solution
|
- A precipitate of .......would be obtained on adding HCl to a solution ...
Text Solution
|