A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ANALYTICAL CHEMISTRY
ERRORLESS |Exercise JEE Section (Integer type Questions)|3 VideosANALYTICAL CHEMISTRY
ERRORLESS |Exercise JEE Section (Only One Choice Answer)|36 VideosANALYTICAL CHEMISTRY
ERRORLESS |Exercise JEE Section (Reasoning Type Questions)|5 VideosALDEHYDES AND KETONES
ERRORLESS |Exercise JEE Section (JEE (Advanced) 2018) Numeric answer type question|1 VideosBIOMOLECULES AND POLYMER
ERRORLESS |Exercise Jee section (Jee (Advanced 2018)) (More than one choice correct answer)|1 Videos
ERRORLESS -ANALYTICAL CHEMISTRY-JEE Section (Comprehension type Question)
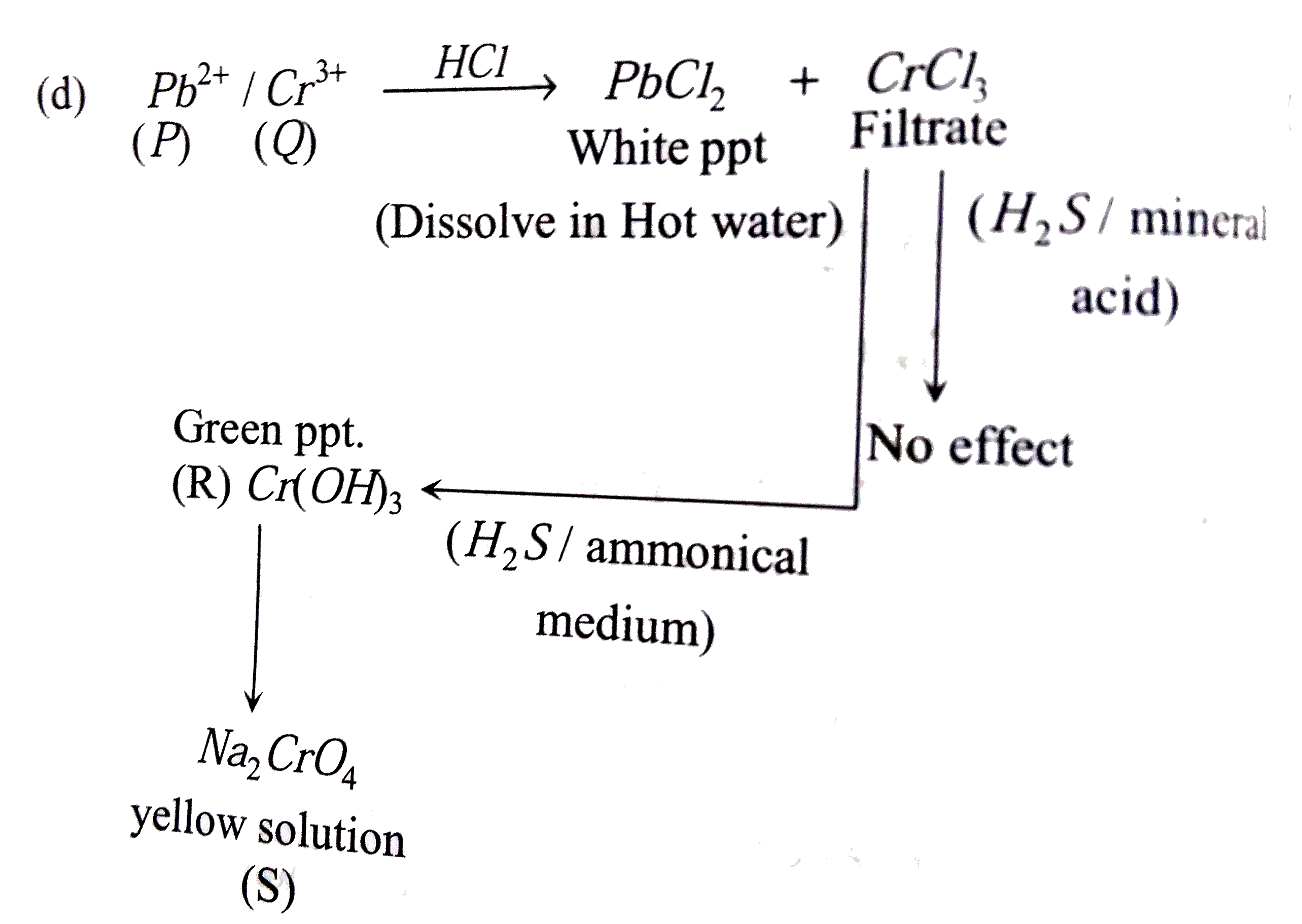
- An aqueous solution of a mixture of two inorganic salts, when treated ...
Text Solution
|
- An aqueous solution of a mixture of two inorganic salts, when treated ...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|