Similar Questions
Explore conceptually related problems
Recommended Questions
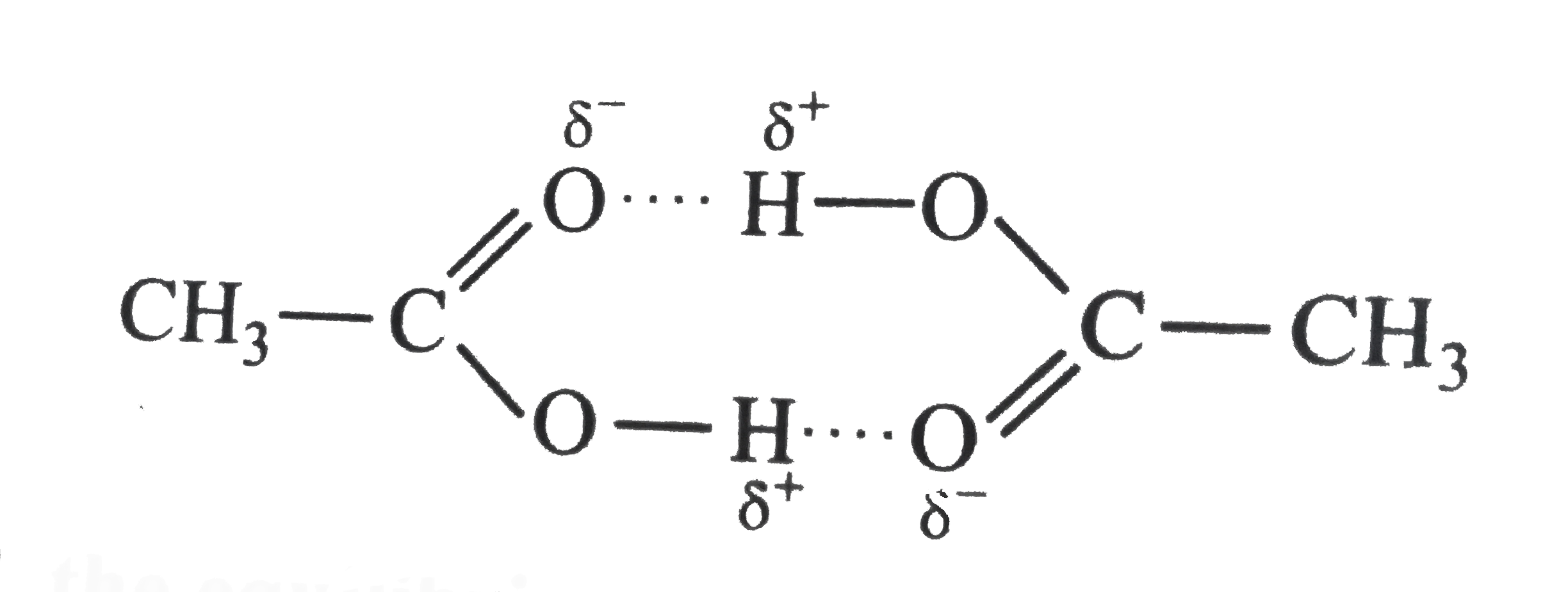
- Acetoc acid CH(3)COOH can form a dimer (CH(3)COOH)(2) in the gas phase...
Text Solution
|
- Acetoc acid CH(3)COOH can form a dimer (CH(3)COOH)(2) in the gas phase...
Text Solution
|
- Arrange the following as stated : 'Increasing order of acidic streng...
Text Solution
|
- Acetic acid forms dimer in vapour phase. The dimer is held together by...
Text Solution
|
- One mole of CH(3)COOH undergo dimerization in vapour state at 127^(@)C...
Text Solution
|
- Arrange the following in order of increasing acidic strength (i) HCOOH...
Text Solution
|
- Calculate the strength of H-bond between F^(-)(g) and CH(3)COOH (g) fr...
Text Solution
|
- The acid strength of the following carboxylic acids increases in the ...
Text Solution
|
- Arrange the following in increasing order of acid strength: ClCH(2)COO...
Text Solution
|