Similar Questions
Explore conceptually related problems
Recommended Questions
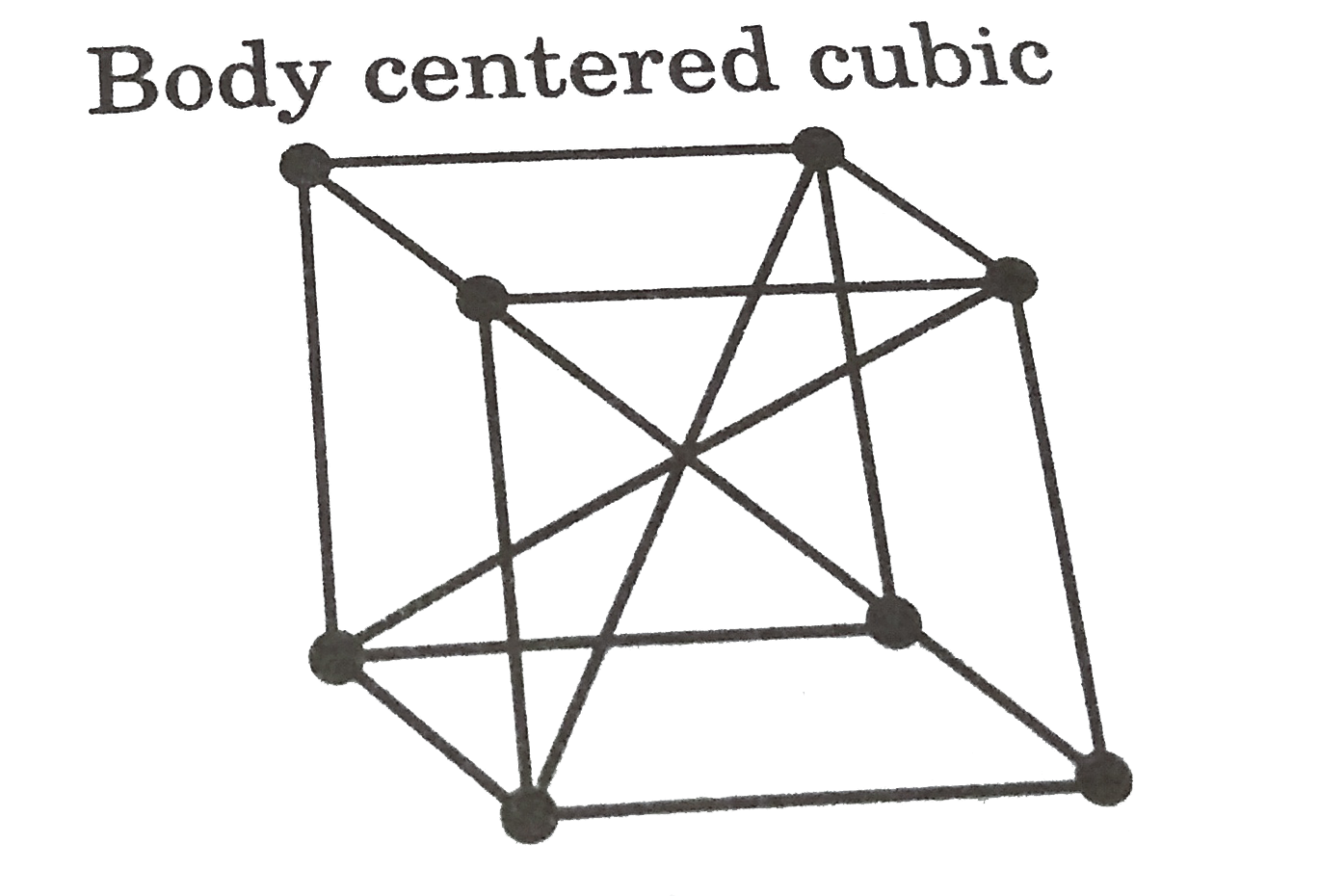
- Metallic sodium has a body-centred cubic unit cell. How many atoms are...
Text Solution
|
- Metallic sodium has a body-centred cubic unit cell. How many atoms are...
Text Solution
|
- How many atoms can be assigned to its unit cell if an element forms (i...
Text Solution
|
- The unit cell of metallic gold is face -centred cubic. How many atoms ...
Text Solution
|
- How many atoms can be assigned to its unit cell if an element forms (i...
Text Solution
|
- Sodium metal crystallises in body centred cubic lattic with cell edge ...
Text Solution
|
- How many particles are there in a body-centred and a face-centred cubi...
Text Solution
|
- A metal has face-centred cubic unit cell. The radius of an atom of the...
Text Solution
|
- In a body centred cubic unit cell, a metal atom at the centre of the c...
Text Solution
|