Similar Questions
Explore conceptually related problems
Recommended Questions
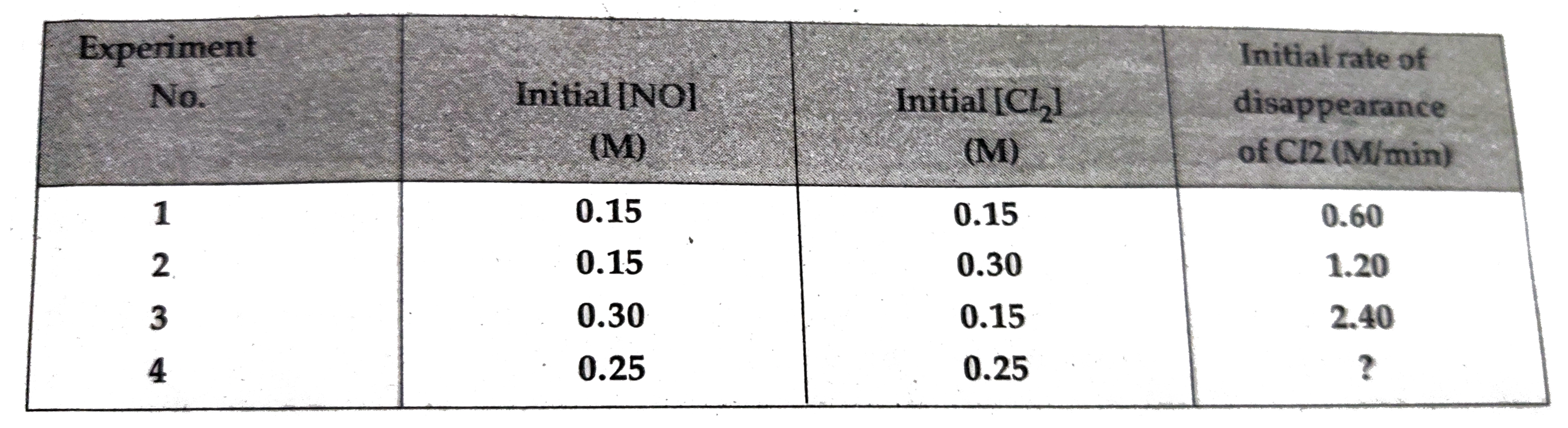
- For the reaction 2NO((g)) +Cl(2(g)) rarr 2NOCl((g)) the following data...
Text Solution
|
- The reaction : 2N(2)O(5)(g) to 2NO(2)(g) + (O(2))(g) was studied and t...
Text Solution
|
- For the reaction 2NO(g) + Cl(2)to2NOCl(g) the following data were ...
Text Solution
|
- For a reaction, Cl(2)(g) + 2NO(g) to 2NOCl(g) ,the rate law is express...
Text Solution
|
- The following data were obtained during the first order decomposition ...
Text Solution
|
- For the reaction 2NO((g)) +Cl(2(g)) rarr 2NOCl((g)) the following data...
Text Solution
|
- निम्न अभिक्रियाओं के लिये साम्य स्थिरांक K(c ) के लिये व्यंजक लिखिए -...
Text Solution
|
- For the reaction Cl(2(g))+2NO((g))rarr 2NOCl(g) The rate law is expres...
Text Solution
|
- In the following reaction: 2NO((g))+Cl(2(g)) hArr 2NOCl((g)) It is see...
Text Solution
|