Similar Questions
Explore conceptually related problems
Recommended Questions
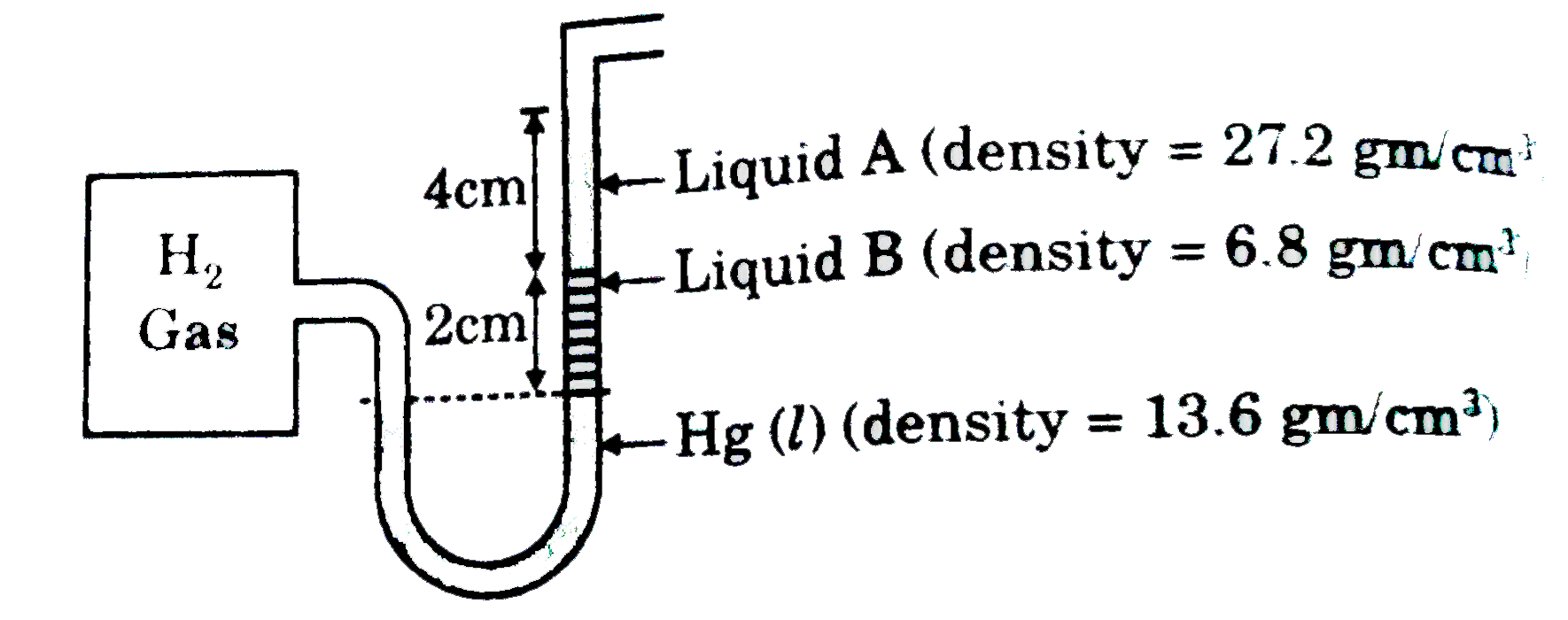
- A contaienr of volume 2 litre contains H(2) gas at 300 K as shown (P("...
Text Solution
|
- What specific name can be given to the following sequence of steps: Hg...
Text Solution
|
- The cell designed as Pt(H(2))|HCl(aq)||Hg(2)Cl(2),0.01 N KCl|Hg has em...
Text Solution
|
- If P(atm) = 0 mm Hg and P(alv) = -2 mm Hg, then
Text Solution
|
- A mixture of C(2)H(2) and C(3)H(8) occupied a certain volume at 80mm H...
Text Solution
|
- A contaienr of volume 2 litre contains H(2) gas at 300 K as shown (P("...
Text Solution
|
- A container consists of 1 mole of liquid water and 1 mole of N(2)(g), ...
Text Solution
|
- A sample of H(2) is collected over H(2)O at 23^(@)C and a pressure of ...
Text Solution
|
- Correct option regarding a container containing 1 mole of a gas in 22....
Text Solution
|