Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of an ideal diatomic gas is taken through the cycle as shown ...
Text Solution
|
- n moles of an ideal gas is taken through a four step cyclic process as...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through the thermodynamic...
Text Solution
|
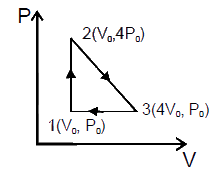
- One mole of helium gas follow cycle 1-2-3-1 shown in the diagram. Duri...
Text Solution
|
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- The straight lines in the figure depict the variations in temperature ...
Text Solution
|
- A gas has been subjected to a cycle of isochoric, isothermal process 1...
Text Solution
|