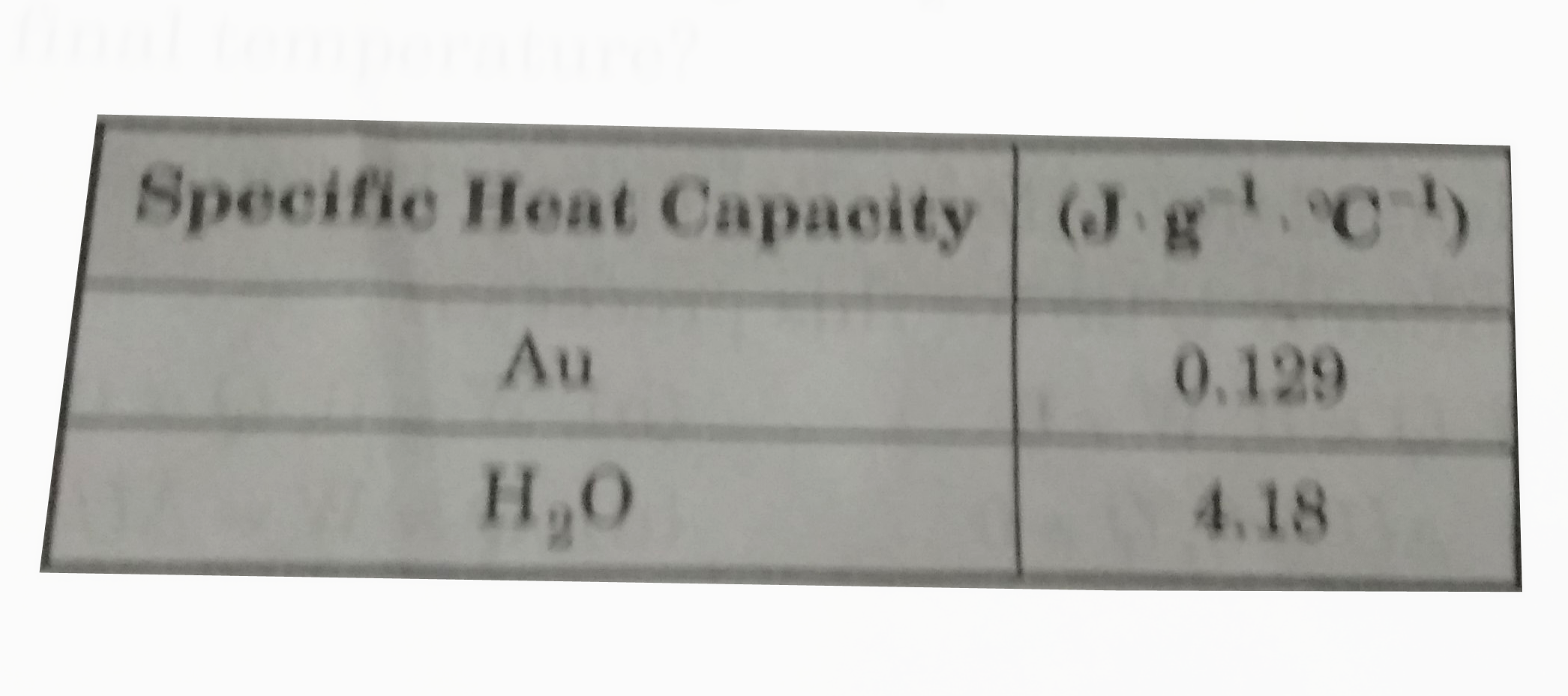Recommended Questions
- A gold ring that weighting 3.81g is heated to 84^(@)Cand placed in 50....
Text Solution
|
- Calculate mass of the final solution obtained on heating a solution co...
Text Solution
|
- A gold ring that weighting 3.81g is heated to 84^(@)C and placed in 50...
Text Solution
|
- An ice cube of unknown mass at 0^(@)C is added to 265g of H(2)O at 25....
Text Solution
|
- 84.12g "of gold at" 120.1^(@)C. Is placed in 106.4g of H(2)Og at 21.4...
Text Solution
|
- A 10.00g piece of metal is heated to 80.00^(@)C and placed in 100.0g ...
Text Solution
|
- The specific heat capacities of three metals are given below. If 1.00g...
Text Solution
|
- A 9.40 g sample of KBr is dissolved in 105 g of H(2)O at 23.6^(@) C in...
Text Solution
|
- A 10.0 g piece of gallium (m=69.7) at 25.0^(@) c is placed in 10.0 g o...
Text Solution
|