Similar Questions
Explore conceptually related problems
Recommended Questions
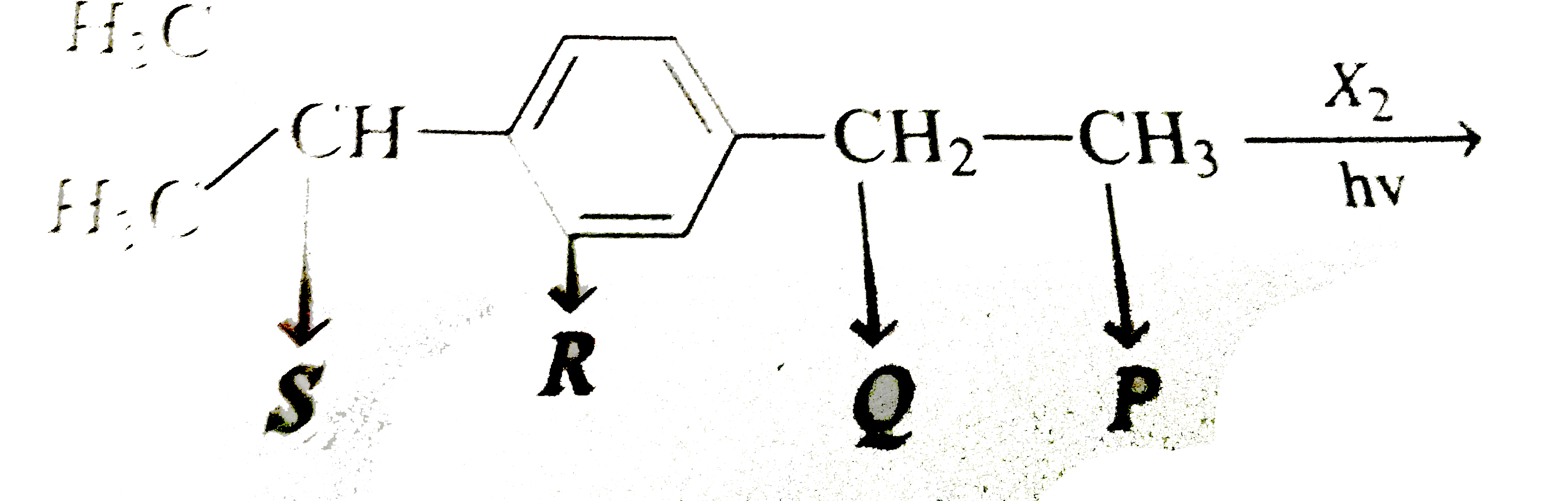
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Free radical substitution chalcogenation is hown by the compunds havin...
Text Solution
|
- Which one of the following is a free - radical substitution reaction ?
Text Solution
|
- The central carbon atom of a free radical contains
Text Solution
|
- मुक्त मूलकों में केंद्रीय कार्बन परमाणु sp^(3) संकरित होता है।
Text Solution
|
- Free Radical Substitution Reactions
Text Solution
|
- Problems on Free Radical Substitution Reactions
Text Solution
|