A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HEAT AND THERMAL PHYSICS
MTG-WBJEE|Exercise WB JEE WORKOUT ( CATEGORY 2: SINGLE OPTION CORRECT TYPE )|14 VideosHEAT AND THERMAL PHYSICS
MTG-WBJEE|Exercise WB JEE WORKOUT ( CATEGORY 2: ONE OR MORE THAN ONE OPTION CORRECT TYPE)|11 VideosGRAVITATION
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 1: Single Option Correct Type|7 VideosKINEMATICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3 : One or More than One Option Correct Type (2 Marks) )|1 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-HEAT AND THERMAL PHYSICS-WB JEE PREVIOUS YEARS QUESTIONS
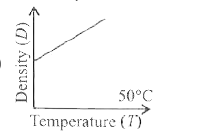
- Which one of the figures gives the temperature dependance of density o...
Text Solution
|
- In which mode of tranmission , the heat waves travel along straight li...
Text Solution
|
- Consider a black body radiation in a cubical box at absolute temperatu...
Text Solution
|
- A small quantity mass m, of water at a temperature theta ("in " ^(@)C)...
Text Solution
|
- Same quantity of ice is filled in each of the two metal containers P a...
Text Solution
|
- A metal rod if fixed rigidly at two ends so as to prevent its hermalex...
Text Solution
|
- A solid at temperature T(1) is kept in an evacuated chamber at tempera...
Text Solution
|
- Three bodies of the same material and having masses m,m and 3m...
Text Solution
|
- If the temperature of the sun (black body) is doubled, the rate of ene...
Text Solution
|
- The temperature of the water of pond is 0^@ C while that of the surro...
Text Solution
|
- A solid rectangular sheet has two different coefficients of linear exp...
Text Solution
|
- The water equivalent of a calorimeter is 10 g and it contains 50 g of ...
Text Solution
|
- Two black bodies A and B have equal surface areas and are maintained a...
Text Solution
|
- A 10 watt electric heater is used to heat a container filled with 0.5 ...
Text Solution
|
- A steam of water flowing horizontally with a speed of 25m ^(s-1) gushe...
Text Solution
|
- Two identical blocks of ice move in opposite directions with equal spe...
Text Solution
|