Similar Questions
Explore conceptually related problems
Recommended Questions
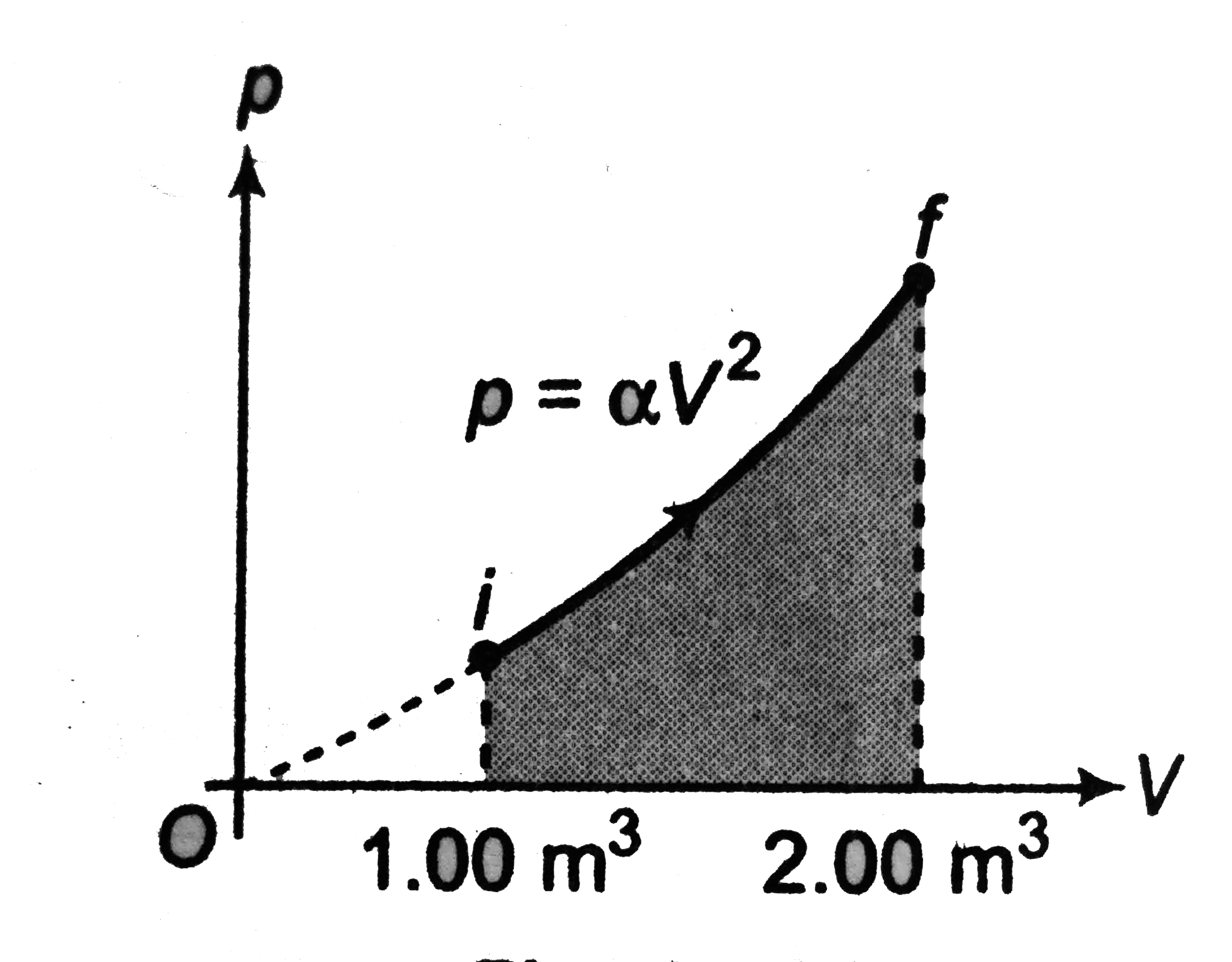
- A sample of ideal gas is expanded to twice its original volume of 1.00...
Text Solution
|
- A sample of ideal gas is expanded to twice its original volume of 1.00...
Text Solution
|
- A sample of an ideal gas is expanded to twice its original volume of 1...
Text Solution
|
- An ideal gas is taken through a quasi-static process described by P = ...
Text Solution
|
- An ideal monatomic gas expands to twice its volume. If the process is ...
Text Solution
|
- A sample of an ideal gas is expanded 1 m^(3) to 3 m^(3) in a reversibl...
Text Solution
|
- A sample of an ideal gas is expanded from 1 dm^(3) to 3 in a reversibl...
Text Solution
|
- A 3 mol sample of an ideal gas at STP expands in an isothermal reversi...
Text Solution
|
- 5 mole of an ideal gas of volume is expanded against vaccum to make it...
Text Solution
|