Similar Questions
Explore conceptually related problems
Recommended Questions
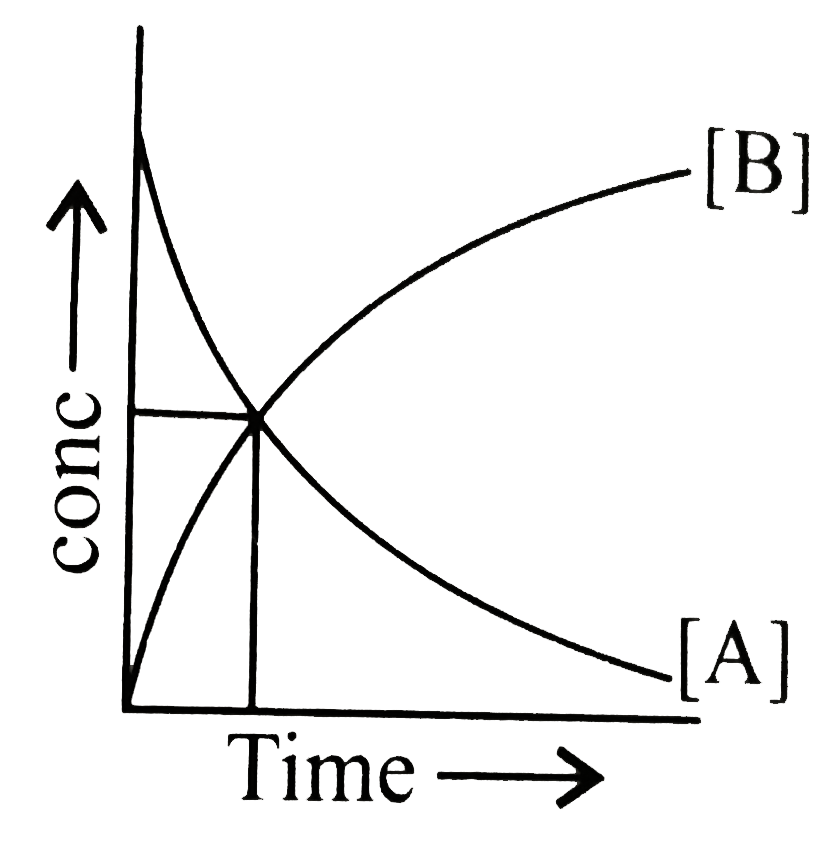
- The accompanying figure depicts a change in concentration of species A...
Text Solution
|
- The accompanying figure depicts a change in concentration of species A...
Text Solution
|
- The figure shows the change in concentration of species A and B as a f...
Text Solution
|
- What quantity is represented by the slope of the dashed line in the ac...
Text Solution
|
- Figure given below shows three velocity-substrate concentration curves...
Text Solution
|
- If the rate of reaction ArarrB doubles on increasing the concentration...
Text Solution
|
- If the reaction, A + 2Brarr 3C+D , which of the following expression d...
Text Solution
|
- Consider the reaction ArarrB . The concentration of both the reactant...
Text Solution
|
- At the point of intersection of the two curves shown for the reaction ...
Text Solution
|