Fuel cells `:` Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen `-` oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst `(` platinum, silver, or metal oxide `).` The electrodes are placed in aqueous solution of `NaOH` . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to `H^(o+)` which is neutralized by `overset(c-)(O)H, i.e.,` anodic reaction.
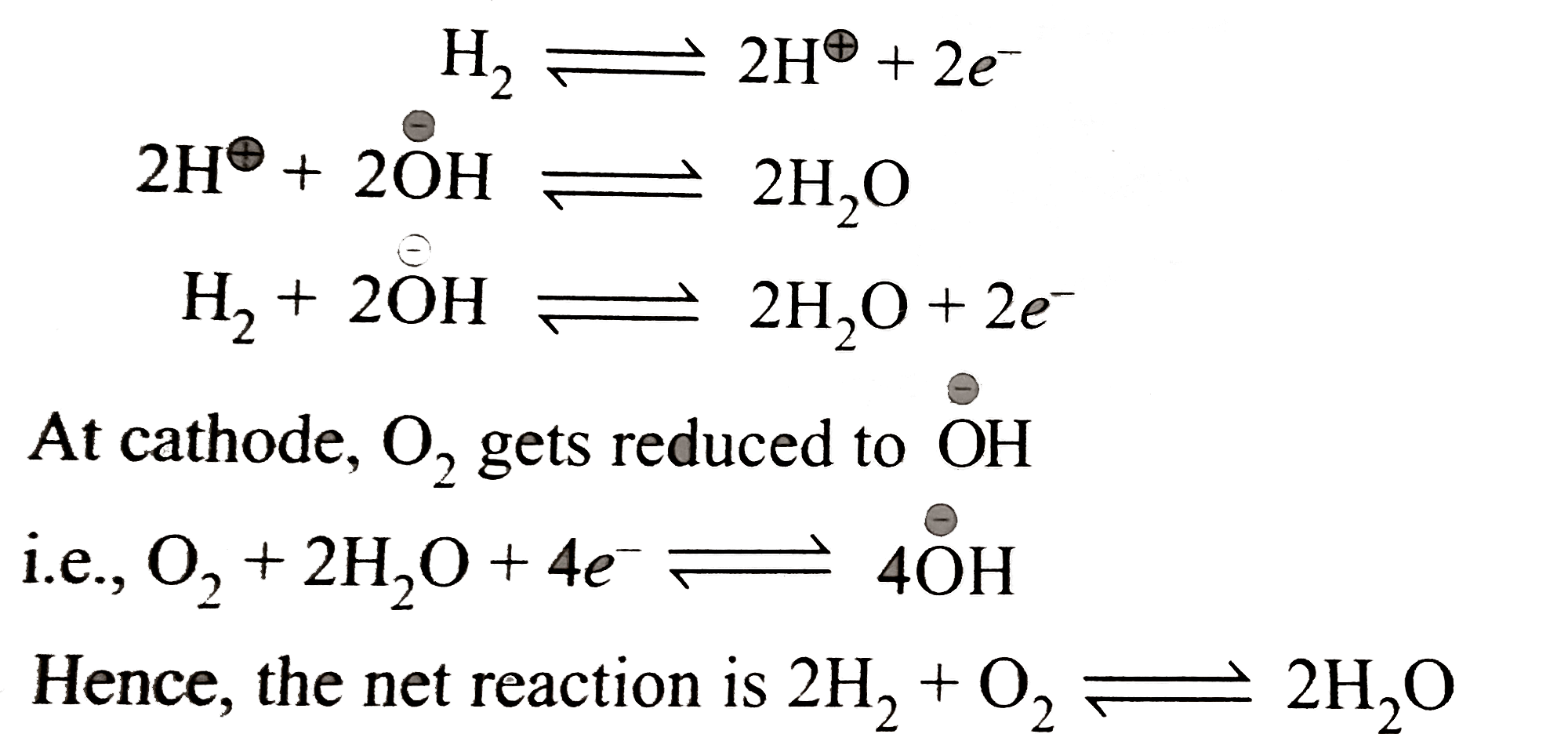
At cathode, `O_(2)` gets reduced to `overset(c-)(O)H`
Hence, the net reaction is
The overall reaction has
`DeltaH=-285.6 kJ mol^(-1)` and `DeltaG=-237.4 kJ mol^(-1)` at `25^(@)C`
A fuel cell is
`I.` A voltaic cell in which continuous supply of fuels are sent at anode to perform oxidation.
`II.` A voltaci cell in which fuels such as `:CH_(4),H_(2),` and `CO` are used up at anode.
`III.` One which involves the reaction of `H_(2)-O_(2)` fuel cell such as `:`
Anode `:2H_(2)O+4overset(c-)(O)H rarr 4H_(2)O(l)+4e^(c-)`
Cathode `: O_(2)+2H_(2)O(l) =4e^(-) rarr 4overset(c-)(O)H`
`IV.` The efficiency of `H_(2)-O_(20` fuel cell is 70 to `75%`
Fuel cells `:` Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen `-` oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst `(` platinum, silver, or metal oxide `).` The electrodes are placed in aqueous solution of `NaOH` . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to `H^(o+)` which is neutralized by `overset(c-)(O)H, i.e.,` anodic reaction.
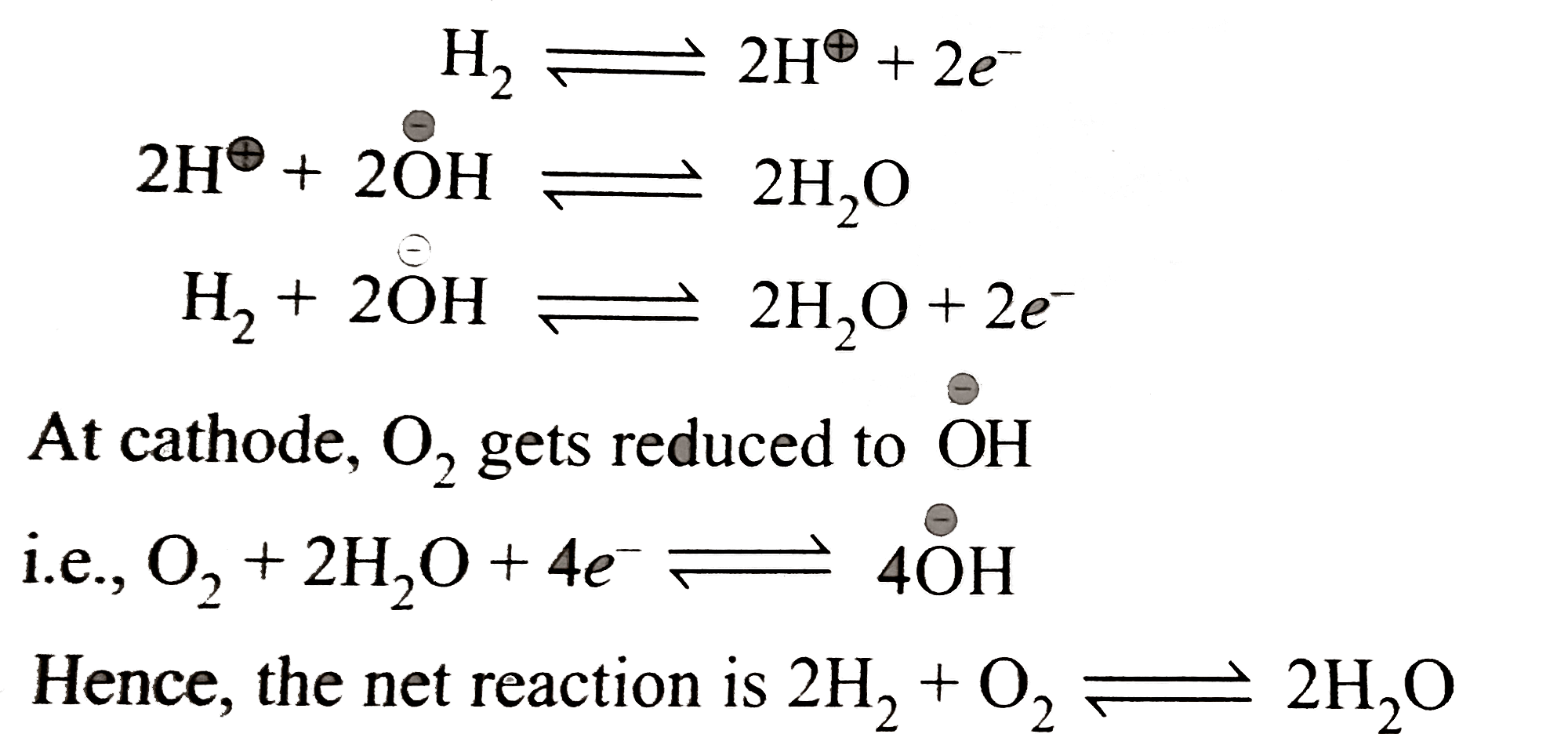
At cathode, `O_(2)` gets reduced to `overset(c-)(O)H`
Hence, the net reaction is
The overall reaction has
`DeltaH=-285.6 kJ mol^(-1)` and `DeltaG=-237.4 kJ mol^(-1)` at `25^(@)C`
A fuel cell is
`I.` A voltaic cell in which continuous supply of fuels are sent at anode to perform oxidation.
`II.` A voltaci cell in which fuels such as `:CH_(4),H_(2),` and `CO` are used up at anode.
`III.` One which involves the reaction of `H_(2)-O_(2)` fuel cell such as `:`
Anode `:2H_(2)O+4overset(c-)(O)H rarr 4H_(2)O(l)+4e^(c-)`
Cathode `: O_(2)+2H_(2)O(l) =4e^(-) rarr 4overset(c-)(O)H`
`IV.` The efficiency of `H_(2)-O_(20` fuel cell is 70 to `75%`
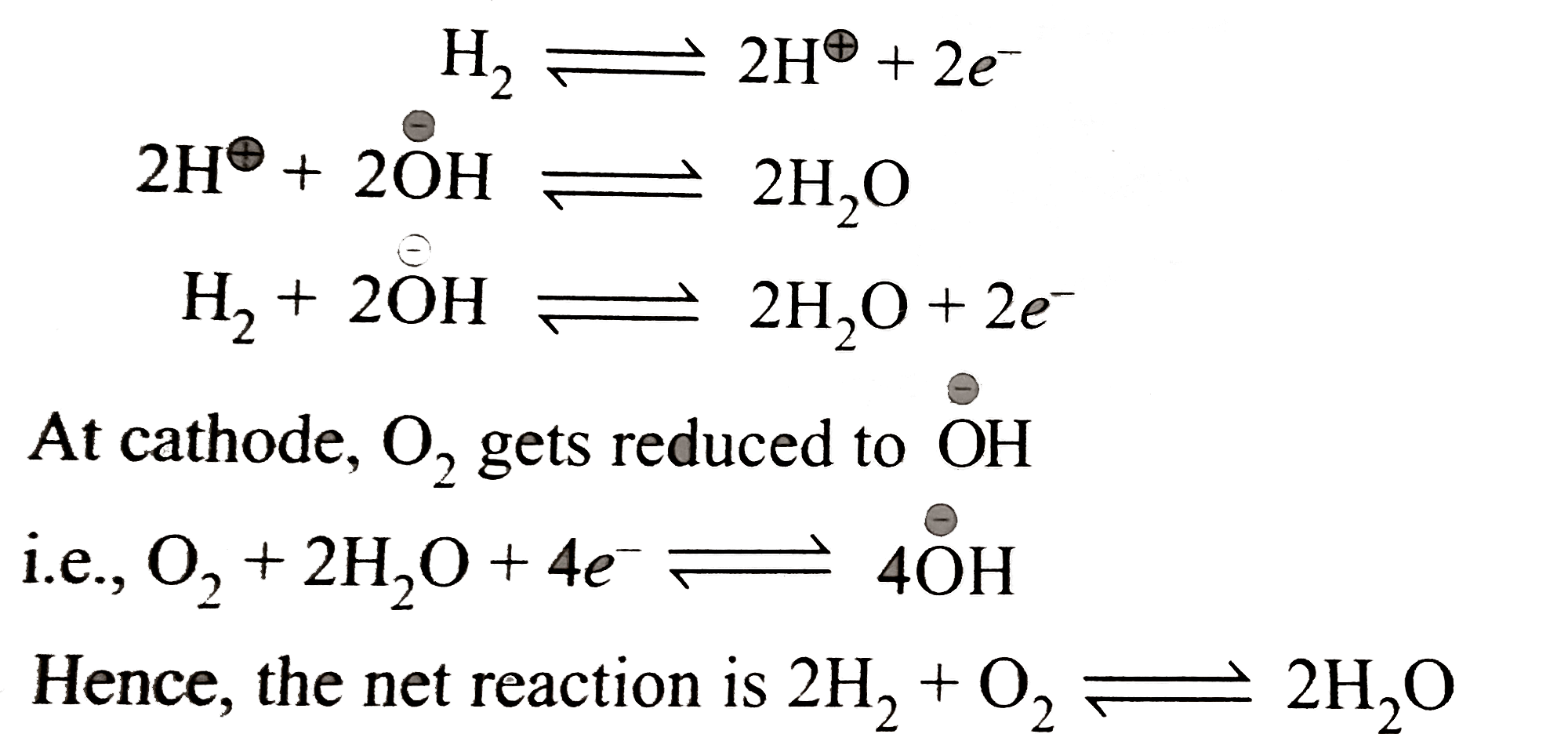
At cathode, `O_(2)` gets reduced to `overset(c-)(O)H`
Hence, the net reaction is
The overall reaction has
`DeltaH=-285.6 kJ mol^(-1)` and `DeltaG=-237.4 kJ mol^(-1)` at `25^(@)C`
A fuel cell is
`I.` A voltaic cell in which continuous supply of fuels are sent at anode to perform oxidation.
`II.` A voltaci cell in which fuels such as `:CH_(4),H_(2),` and `CO` are used up at anode.
`III.` One which involves the reaction of `H_(2)-O_(2)` fuel cell such as `:`
Anode `:2H_(2)O+4overset(c-)(O)H rarr 4H_(2)O(l)+4e^(c-)`
Cathode `: O_(2)+2H_(2)O(l) =4e^(-) rarr 4overset(c-)(O)H`
`IV.` The efficiency of `H_(2)-O_(20` fuel cell is 70 to `75%`
Similar Questions
Explore conceptually related problems
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C If the cell voltage is 1.23V for the H_(2)-O_(2) fuel cell and for the half cell :
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C If the cell voltage is 1.23V for the H_(2)-O_(2) fuel cell and for the half cell :
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C If the cell voltage is 1.23V for the H_(2)-O_(2) fuel cell and for the half cell :
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C What is the value of DeltaS^(c-) for the fuel cell at 25^(@)C ?
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C What is the value of DeltaS^(c-) for the fuel cell at 25^(@)C ?
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C What is the value of DeltaS^(c-) for the fuel cell at 25^(@)C ?
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C Suppose the concentration of hydroxide ioin in the cell is doubled, then the cell voltage will be
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C Suppose the concentration of hydroxide ion in the cell is doubled, then the cell voltage will be
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C Suppose the concentration of hydroxide ioin in the cell is doubled, then the cell voltage will be
Fuel cells : Fuel cells are galvanic cells in which the chemical energy of fuel cell is directly converted into electrical energy. A type of fuel cell is a hydrogen - oxygen fuel cell. It consists of two electrodes made up of two porous graphite impregnated with a catalyst ( platinum, silver, or metal oxide ). The electrodes are placed in aqueous solution of NaOH . Oxygen and hydrogen are continuously fed into the cell. Hydrogen gets oxidized to H^(o+) which is neutralized by overset(c-)(O)H, i.e., anodic reaction. At cathode, O_(2) gets reduced to overset(c-)(O)H Hence, the net reaction is The overall reaction has DeltaH=-285.6 kJ mol^(-1) and DeltaG=-237.4 kJ mol^(-1) at 25^(@)C What is the value of DeltaS^(c-) for the fuel cell at 25^(@)C ? a. -1600 J K^(-1) b. -160 J K^(-1) c. 160 J K^(-1) d. 1600 J K^(-1)
Recommended Questions
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- ईधन सेल क्या हैं। हाइड्रोजन –ऑक्सीजन ईधन सेल का वर्णन संक्षेप में कीज...
Text Solution
|
- ईंधन सेल में ईंधन ऊर्जा को विद्युत ऊर्जा में बदला जाता है।
Text Solution
|
- What are fuel cells ? Discuss briefly hydrogen-oxygen fuel cell ?
Text Solution
|
- What are fuel cells ? Write the electrode reactions of a fuel cell whi...
Text Solution
|