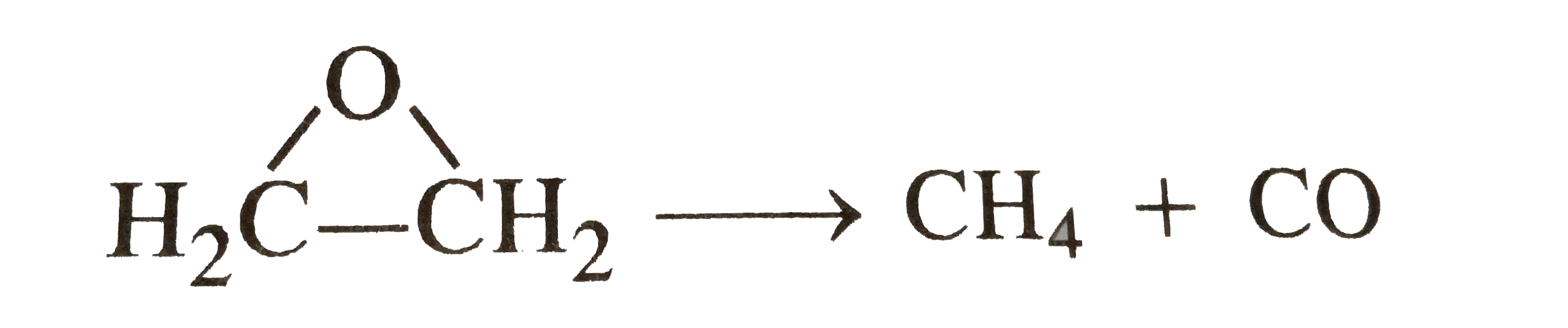Similar Questions
Explore conceptually related problems
Recommended Questions
- The rate constant for the first order decomposition of ethylene oxide ...
Text Solution
|
- The rate constant for the first order decompoistion of a certain react...
Text Solution
|
- Rate constant for a reaction varies with temperature as, ln K(sec^(-1)...
Text Solution
|
- The rate constant for the first order decomposition of ethylene oxide ...
Text Solution
|
- Rate constant for the decomposition of ethylene oxide into CH(4) and C...
Text Solution
|
- The rate constant for the first order decompoistion of a certain react...
Text Solution
|
- For a first order reaction, the rate constant (k) is 1.25 X 104 repres...
Text Solution
|
- किसी अभिक्रिया के प्रथम कोटि अपघटन के लिए वेग स्थिरांक निम्न समीकरण द्...
Text Solution
|
- किसी अभिक्रिया के प्रथम कोटि अपघटन के लिए वेग स्थिरांक निम्न समीकरण द्...
Text Solution
|