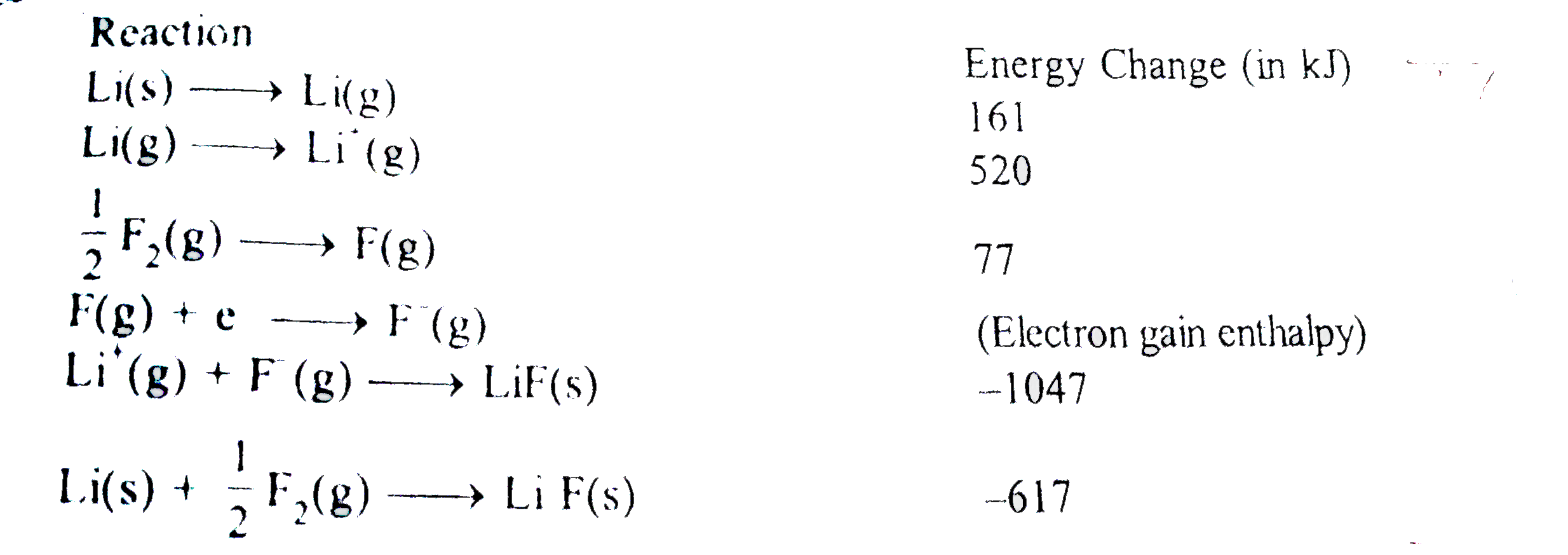Similar Questions
Explore conceptually related problems
Recommended Questions
- Given Based on data provided, the value of electron gain enthalpy...
Text Solution
|
- Ionisation of energy F^(ɵ) is 320 kJ mol^(-1) . The electronic gain en...
Text Solution
|
- Why fluorine has lesser electron gain enthalpy than chlorine?
Text Solution
|
- The negative value of electron gain enthalpy is less for fluorine than...
Text Solution
|
- Ionisation energy of F^(-) is +320 mol^(-1) . The electron gain enthal...
Text Solution
|
- Given Based on data provided, the value of electron gain enthalpy of f...
Text Solution
|
- The electron gain enthalpy ( in kJ//mol ) of fluorine, chlorine,...
Text Solution
|
- Electron gain enthalpy of fluorine is less than that of chlorine - exp...
Text Solution
|
- The negative value of electron gain enthalpy is less for fluorine than...
Text Solution
|