Similar Questions
Explore conceptually related problems
Recommended Questions
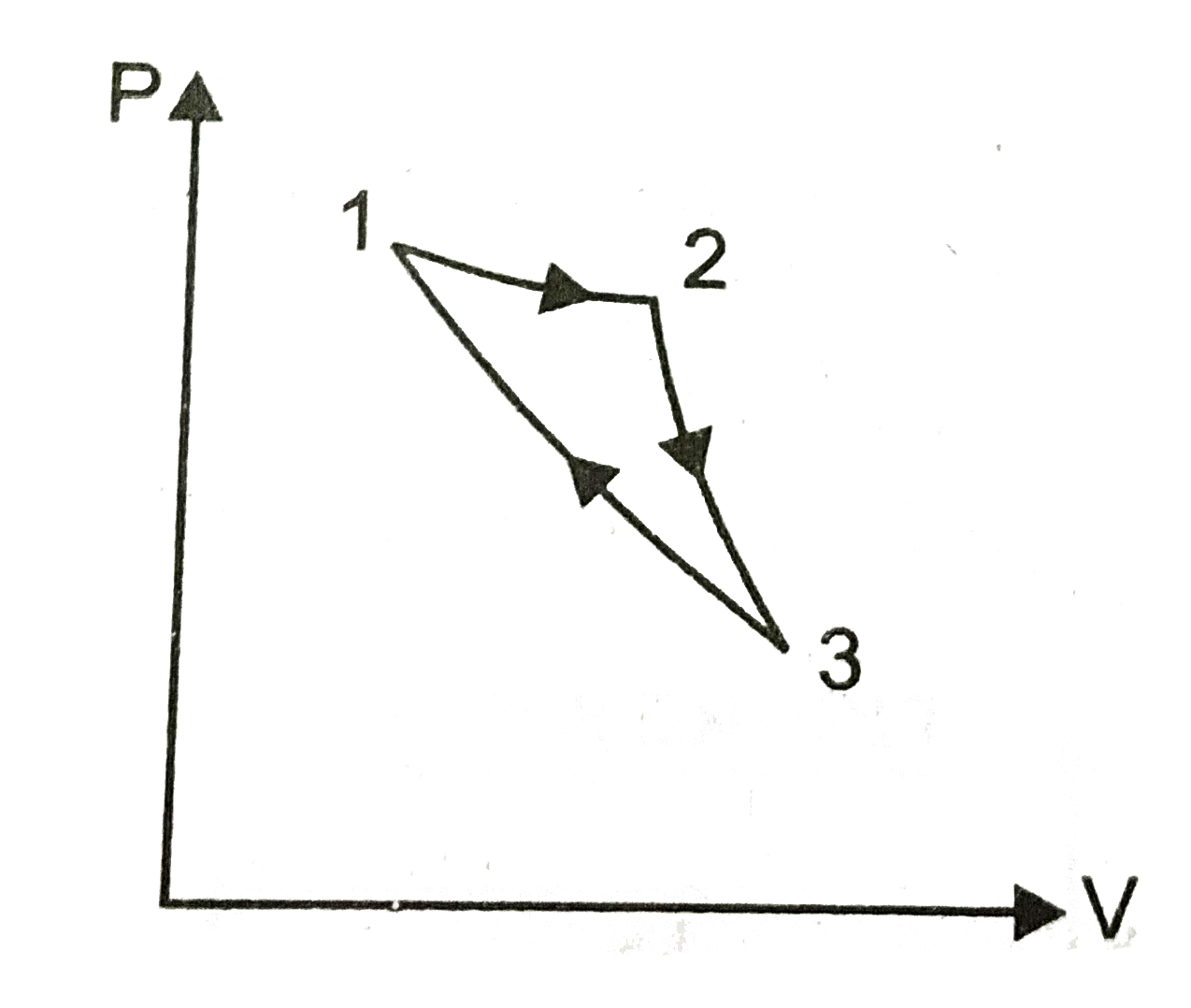
- Consider a cycle followed by an engine, (figure) 1 to 2 is isothe...
Text Solution
|
- Assertion: Isothermal and adiabatic, two processes are shown on p-V di...
Text Solution
|
- Consider a cycle followed by an engine, (figure) 1 to 2 is isothermal ...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- Consider a cycle followed by an engine (figure.) 1 or 2 is isoth...
Text Solution
|
- Isothermal Process and Adiabatic Process.
Text Solution
|
- Write the mathmatical expression of the First Law of Thermodynamics fo...
Text Solution
|
- Differentiate between isothermal and adiabatic process.
Text Solution
|