Similar Questions
Explore conceptually related problems
Recommended Questions
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure insi...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure insi...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure insi...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure insi...
Text Solution
|
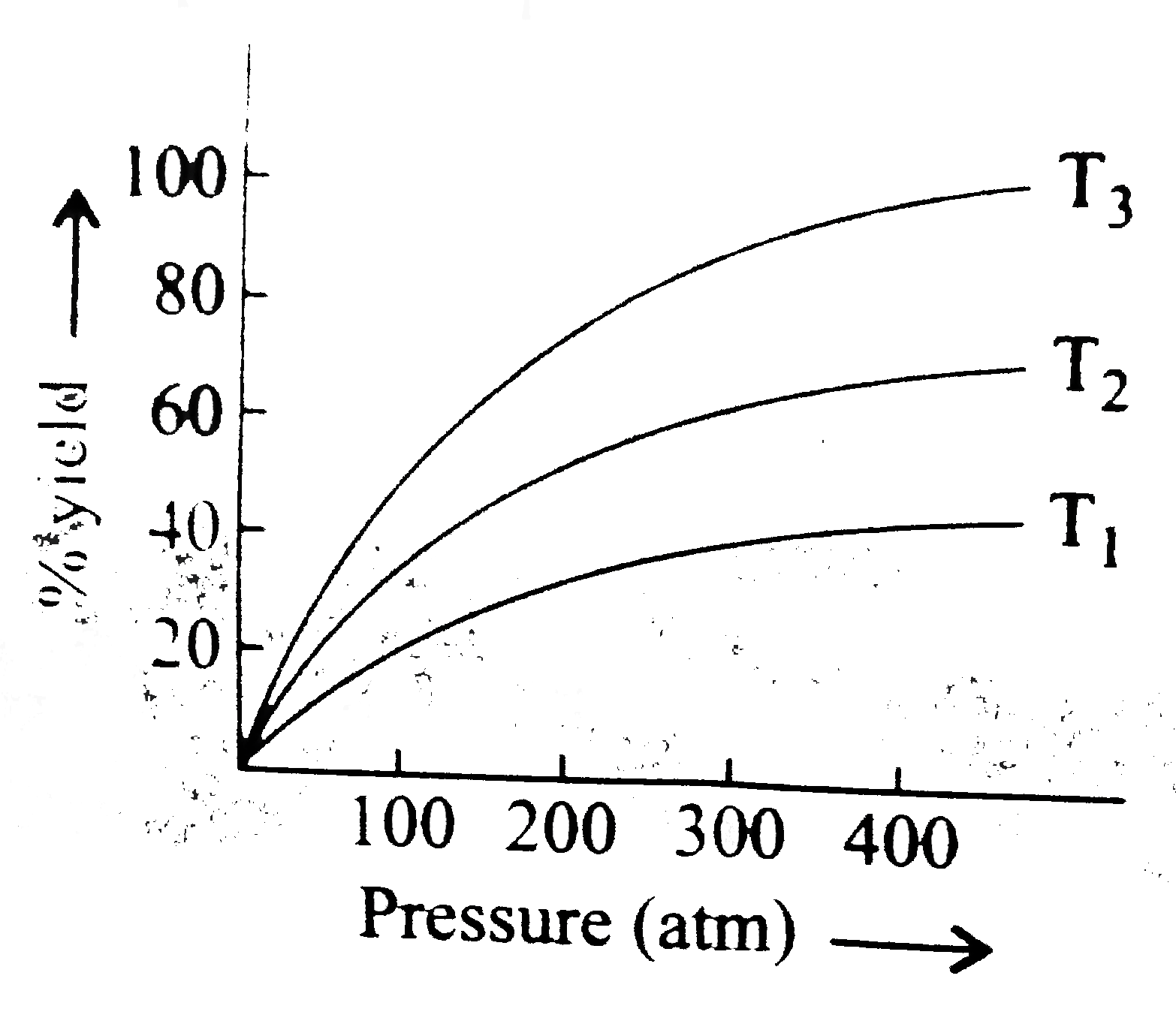
- The % yield of ammonia as a function of time in the reaction, N(2)(g)+...
Text Solution
|
- The prepation of SO(3)(g) by reaction SO(2)(g)+(1)/(2)O(2)(g)hArrSO(3)...
Text Solution
|
- अभिक्रिया, N(2)(g)+3H(2)(g)leftrightarrow2NH(3)(g), DeltaH<0 में अमोनि...
Text Solution
|