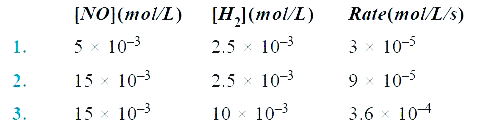Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- Write the relation between kp and Kc for the following reactions. (i...
Text Solution
|
- निम्न अभिक्रिया के लिये Delta S^@ का चिन्ह/मान प्रस्तावित कीजिये ...
Text Solution
|
- Predict the sign of entropy change is the following reactions 2H2(g)+O...
Text Solution
|
- Write the relation between kp and Kc for the following reactions. (i) ...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|