Similar Questions
Explore conceptually related problems
Recommended Questions
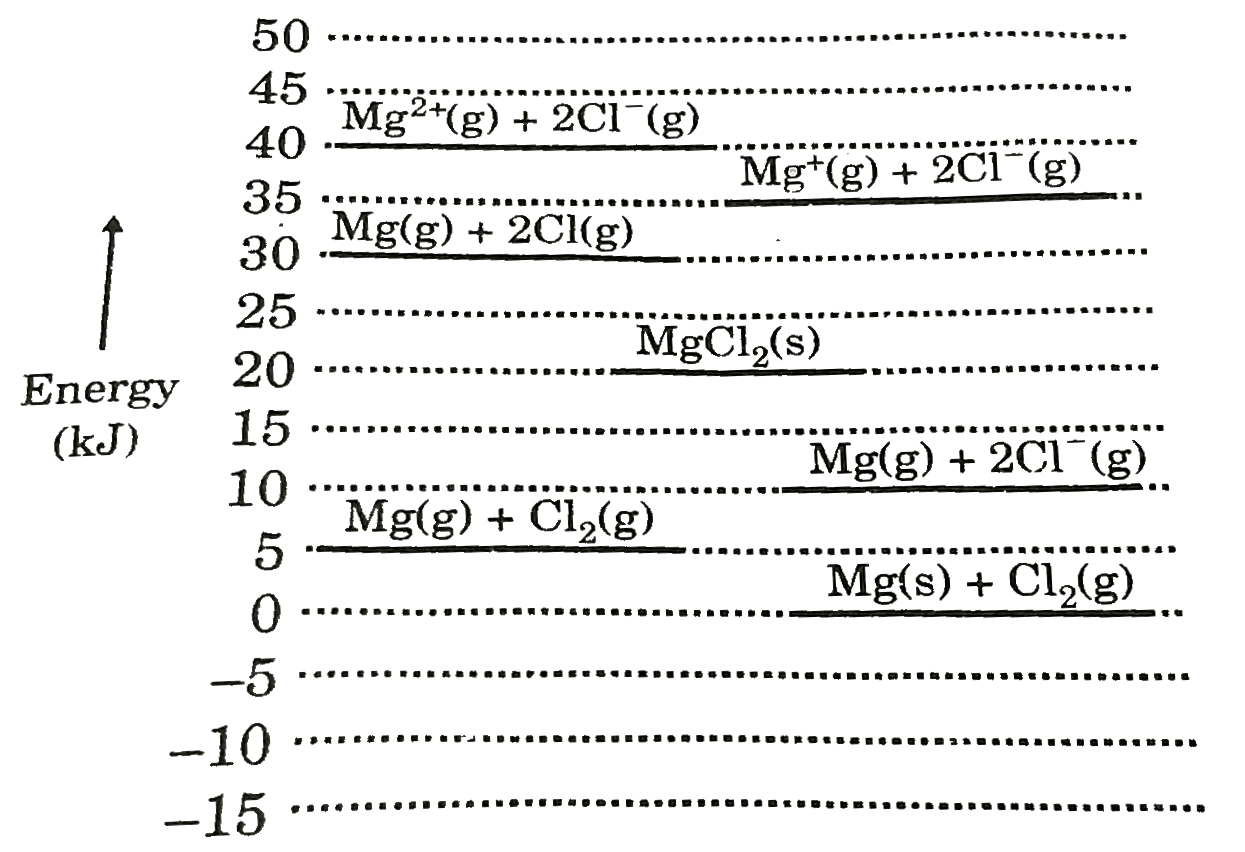
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Assertion:Born-Haber cycle is based on Hess's law. Reason: Lattice e...
Text Solution
|
- प्रक्कथन : बॉर्न-हैबर चक्र, हैस के नियम पर आधारित है। कारण : बॉर्न-...
Text Solution
|
- Explain the Born-Haber Cycle. OR Lattice Enthalpy.
Text Solution
|
- Ionic Bond |Ionisation/Electron gain Enthalpy#!#Lattice Enthalpy #!#Bo...
Text Solution
|
- a) Define lattice enthalpy of an ionic compound. b) What is Born-Hab...
Text Solution
|