Similar Questions
Explore conceptually related problems
Recommended Questions
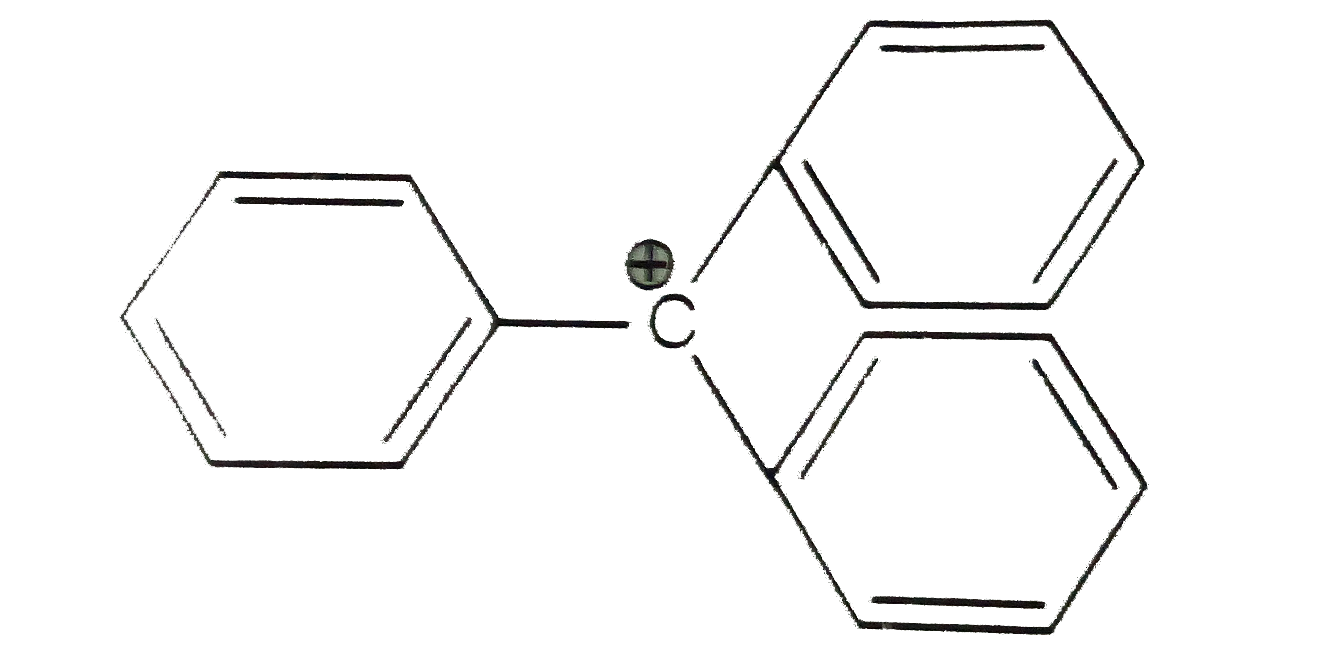
- The structure of triphenylmethyl cation is given below. This is very s...
Text Solution
|
- On reaction with acid, 4-pyrone gives a very stable cationic product. ...
Text Solution
|
- The structure of triphenylmethyl cation is given below. This is very s...
Text Solution
|
- Among the given cation,s the most stable carbonium ions is ?
Text Solution
|
- Among the given cation,s the most stable carbonium ions is ?
Text Solution
|
- The structure of triphenylmethyl cation is given below . This very sta...
Text Solution
|
- "Stability of carbocations depends upon the electron releasing inducti...
Text Solution
|
- 'Stability of carbocations depends upon the electron releasing inducti...
Text Solution
|
- Which carbonium ion is more stable in the given cations
Text Solution
|