Similar Questions
Explore conceptually related problems
Recommended Questions
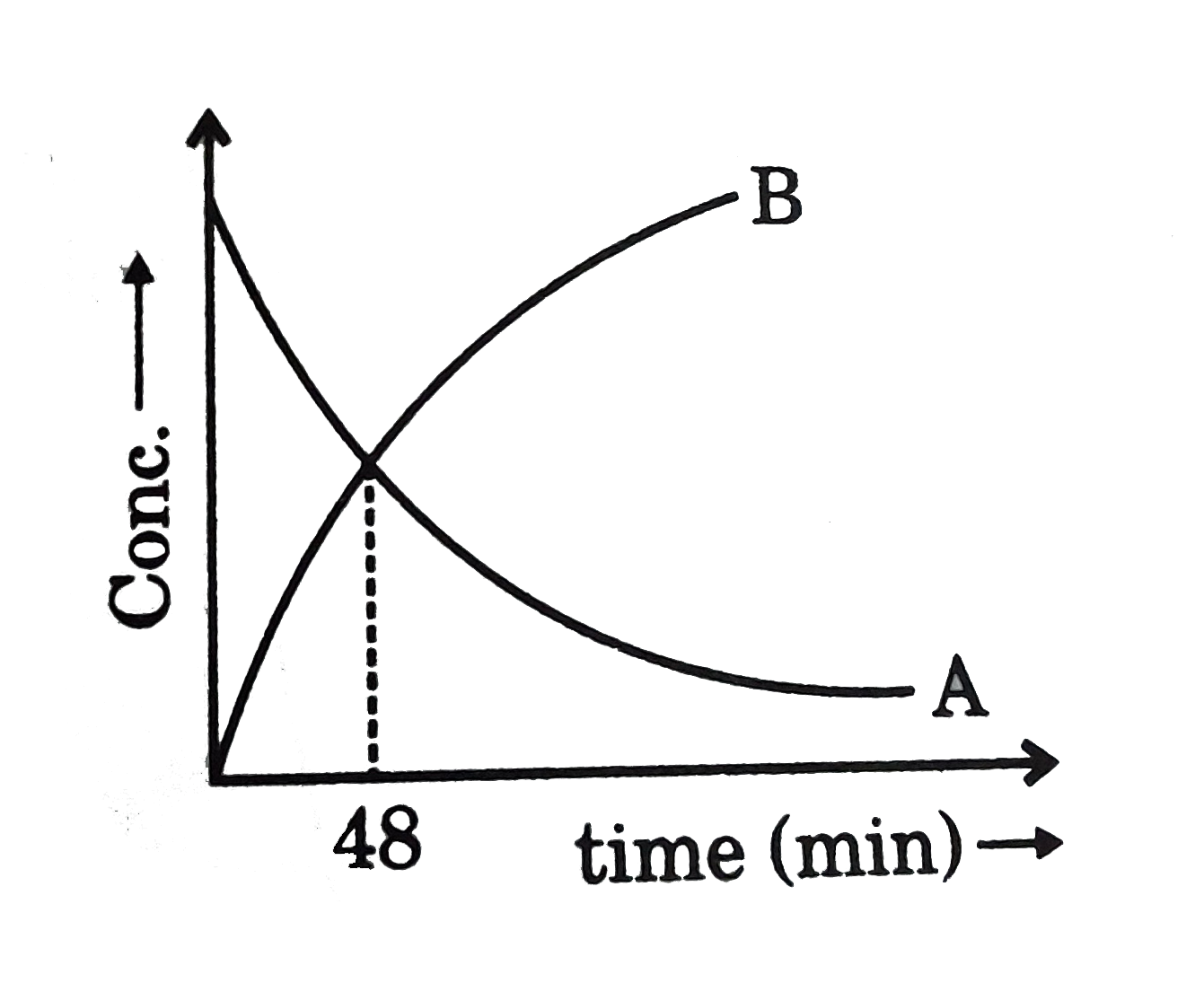
- For a 1st order reaction, nA rarr B whose concentration vs time curve ...
Text Solution
|
- For the zero order reaction A rarr B+C, initial concentration of A is ...
Text Solution
|
- For a 1st order reaction, nA rarr B whose concentration vs time curve ...
Text Solution
|
- For a first order reaction , nAtoB whose concentration vs time curve i...
Text Solution
|
- For a reaction A + B rarr product, the rate of the reaction was double...
Text Solution
|
- At particular concentration, the half life of the reaction is 100 minu...
Text Solution
|
- At particular concentration , the half life of the reaction is 100 min...
Text Solution
|
- Rate of chemical reaction nA rarr Product, is doubled when the concent...
Text Solution
|
- 99% of a 1st order reaction completed in 2.303 minutes. What is the ra...
Text Solution
|