Similar Questions
Explore conceptually related problems
Recommended Questions
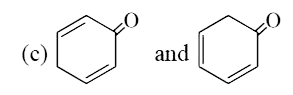
- Which of the following pairs has higher resonance energy :
Text Solution
|
- In which of the following pairs, the first member has higher first ion...
Text Solution
|
- In which of the followig pairs II^(nd) compound has less resonance ene...
Text Solution
|
- In which of the following pairs II^(nd) compound has higher resonance ...
Text Solution
|
- कौन-से अणु का अनुनाद स्थयित्व अधिक है ?
Text Solution
|
- In which of the following pair's first compound is having higher reso...
Text Solution
|
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy: and CH(2) = ...
Text Solution
|