Similar Questions
Explore conceptually related problems
Recommended Questions
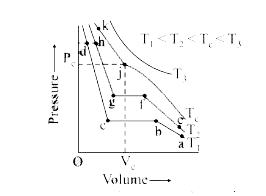
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperature are represented in ...
Text Solution
|
- The isothermal diagram of a gas at three different temperatures T(1),T...
Text Solution
|
- Figure shows the isotherms of fixed mass of an ideal gas at three temp...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|