Similar Questions
Explore conceptually related problems
Recommended Questions
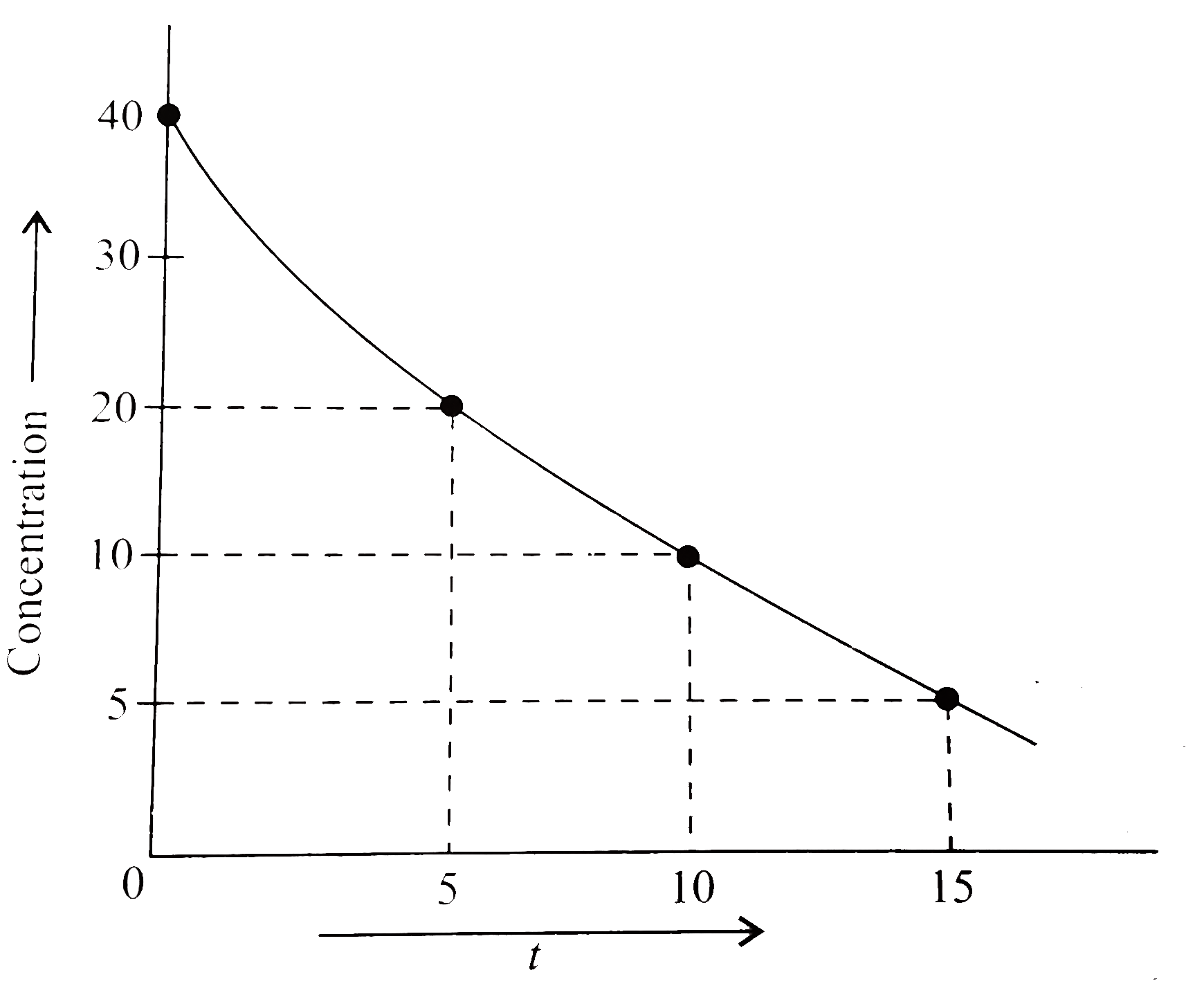
- For a reaction , a graph was plotted between reactant concentration c ...
Text Solution
|
- In the decompoistion of N(2)O(5), the plot between the reciprocal of c...
Text Solution
|
- For a reaction , a graph was plotted between reactant concentration c ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- For a zero order reaction the plot of concentration of reactant versus...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- Analyse the given graph,drawn between concentration of reactant vs. ti...
Text Solution
|
- What will be the nature of the graph showing the concentration of the ...
Text Solution
|
- Plot a graph of concentration of a reactant at time t, [ (a-x)] agains...
Text Solution
|