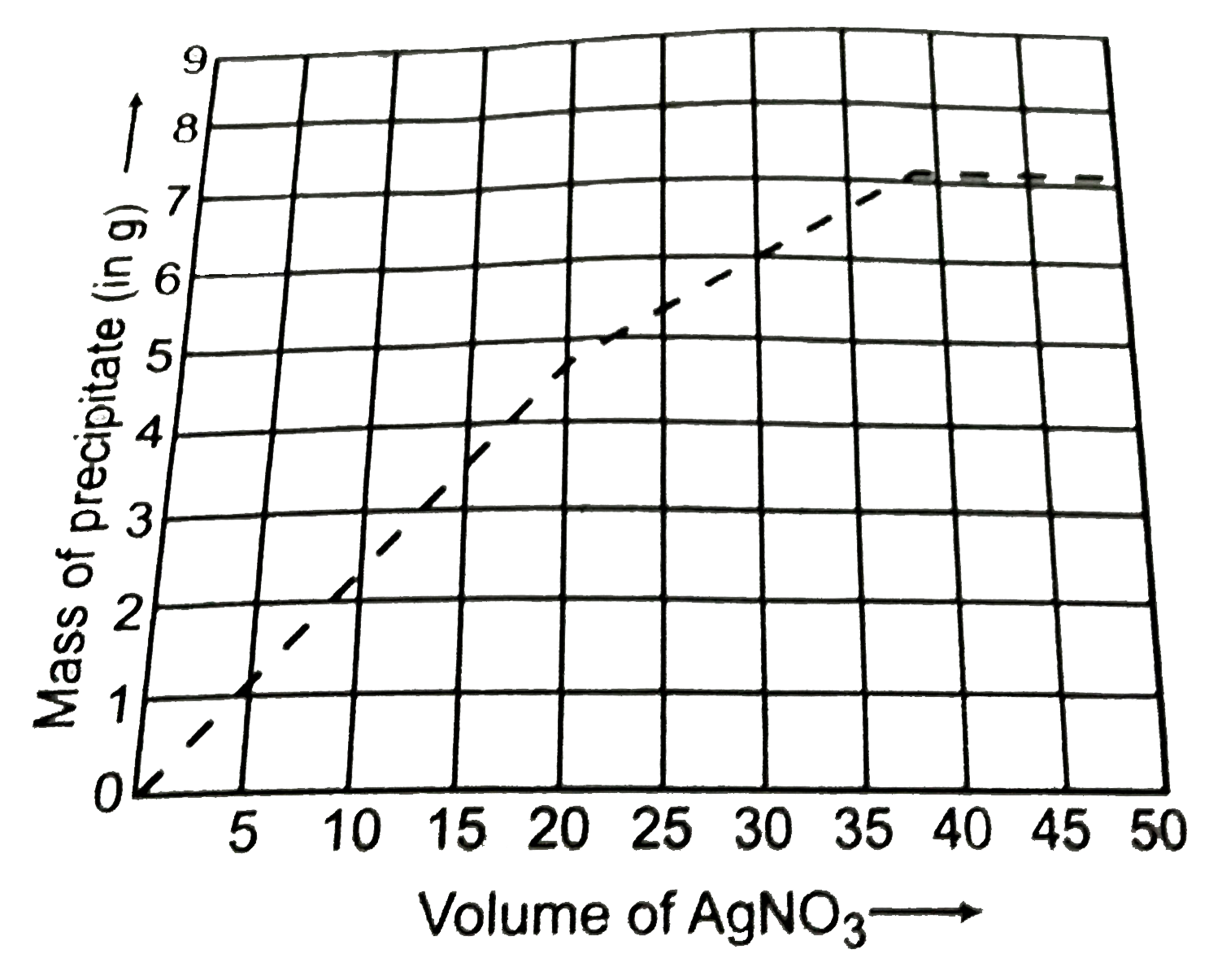
Similar Questions
Explore conceptually related problems
Recommended Questions
- 4.0 g of a mixture of NaCl and an unknown metal iodide MI(2) was disso...
Text Solution
|
- What weight of AgCl will be precipitated when a solution containing 4....
Text Solution
|
- Silver nitrate solution is gradually added to an aqueous conjuaining 0...
Text Solution
|
- Silver nitrate solution is gradually added to an aqueous solution cont...
Text Solution
|
- 4.0 g of a mixture of NaCl and an unknown metal iodide MI(2) was disso...
Text Solution
|
- 4.0 g of a mixture of NaCl and an unknown metal iodide MI(2) was disso...
Text Solution
|
- 4.0 g of a mixture of NaCl and an unknown metal iodide MI(2) was disso...
Text Solution
|
- Silver nitrate solution is gradually added to an aqueous solution cont...
Text Solution
|
- AgNO(3) (aq) was added to an aqueous KCl solution gradually and the co...
Text Solution
|