Similar Questions
Explore conceptually related problems
Recommended Questions
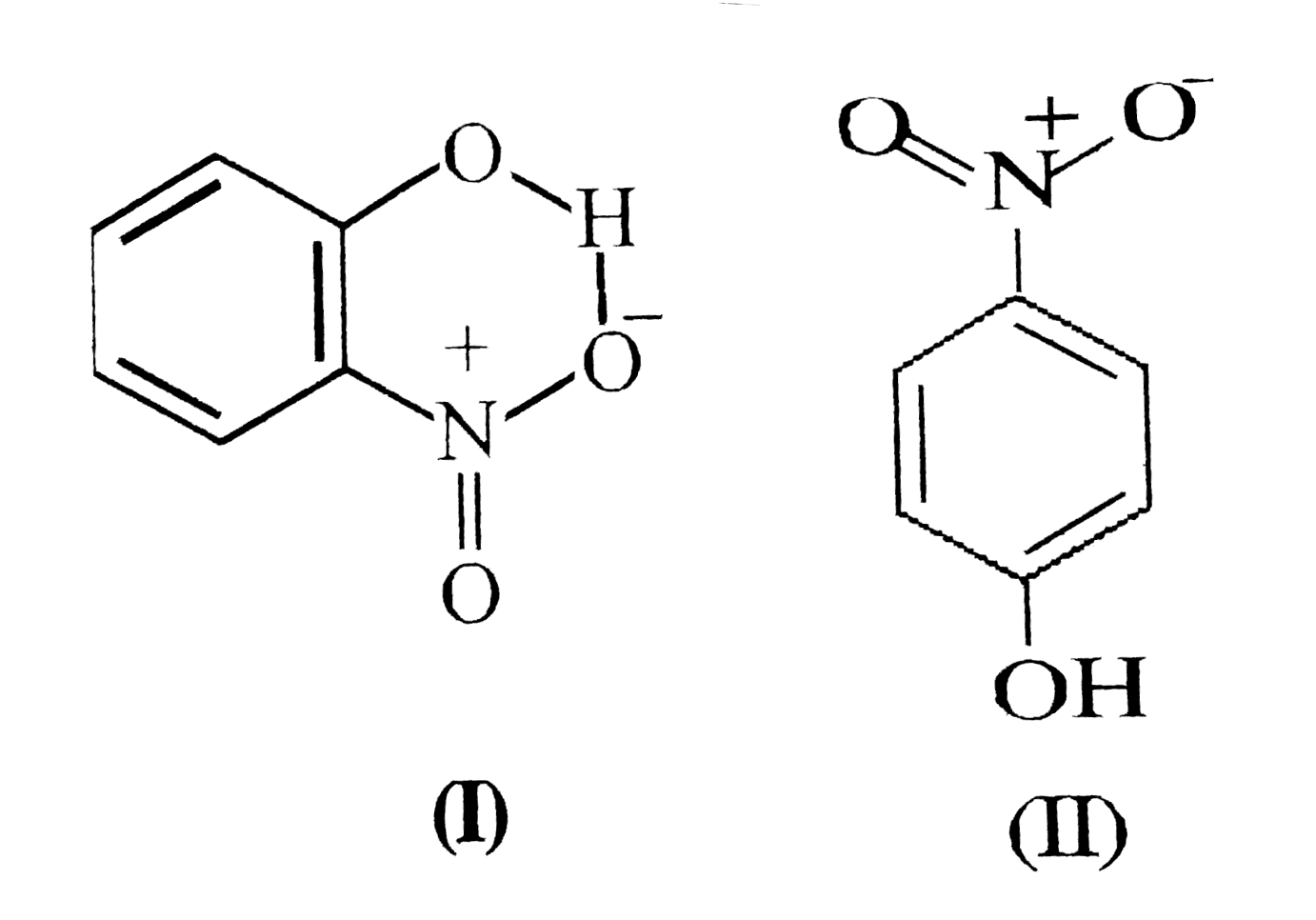
- Structures of molecules of two cumpoundes are given: (a) Which of th...
Text Solution
|
- In Which of the following compound are intermolecular hydrogen bonds n...
Text Solution
|
- Structures of molecules of two compounds are given: (a) Which of the t...
Text Solution
|
- Structures of molecules of two compounds are given below. a) Which of ...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- The hydrogen bond is an electrostatic attractive force between covalen...
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|